FDA gives nod to Stryker’s Neuroform Atlas Stent System
por John R. Fischer, Senior Reporter | November 15, 2017
Cardiology
Stents
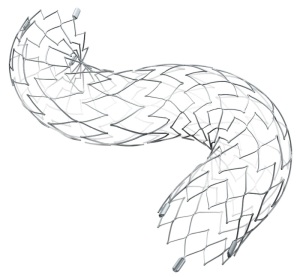
Neuroform Atlas Stent System
Stryker Corporation is set to begin commercialization of its Neuroform Atlas Stent System in the U.S., following FDA approval.
The device is designed to be used in conjunction with neurovascular embolic coils to treat wide neck, intracranial, and saccular aneurysms.
“These wide neck aneurysms can often be difficult to treat with coils alone, due to their anatomy,” Mark Paul, president of the neurovascular division at Stryker, told HCB News. “The Neuroform Atlas is placed across the aneurysm neck during the procedure, enabling the coils to be deployed and remain inside the aneurysm.”
Neuroform Atlas was cleared for marketing under a humanitarian device exemption (HDE), a designation awarded to devices used to diagnose and treat conditions that affect fewer than 8,000 people throughout the United States each year. An estimated 6 million people nationwide have an unruptured brain aneurysm of which roughly 25,000 are treated with endovascular or surgical treatments each year. Wide neck aneurysms account for less than 10 percent of treated unruptured aneurysms.
The system is a small nitinol stent that is used alongside metal coils to pack aneurysms in the brain. The stent, positioned through a small tube, is delivered across the neck of the aneurysm to support and pack it.
The hybrid cell stent design provides improvement to wall apposition, ease of use, deployment accuracy and catheter re-entry in the most challenging cases. Deployment accuracy is high, due to its low foreshortening.
Neuroform Atlas is also compatible with the lowest profile coiling microcatheters on the market, enabling physicians to reach smaller, more distal vessels within the neurovasculature, which may not be accessible with larger-bore catheters that are required to deliver other treatment devices.
“This can potentially reduce the number of steps and equipment in each procedure by allowing the physician to use the same microcathether to deliver the Neuroform Atlas and the embolic coils,” said Paul.
Stryker completed enrollment in the Neuroform Atlas US IDE clinical trial for the anterior arm earlier this year. Enrollment continues for the posterior arm. The trial consists of the largest data set on adjunctive stent use with intracranial aneurysm coiling.
The system is currently on sale in 47 countries, including parts of Europe, Asia, and South America.
The device is designed to be used in conjunction with neurovascular embolic coils to treat wide neck, intracranial, and saccular aneurysms.
“These wide neck aneurysms can often be difficult to treat with coils alone, due to their anatomy,” Mark Paul, president of the neurovascular division at Stryker, told HCB News. “The Neuroform Atlas is placed across the aneurysm neck during the procedure, enabling the coils to be deployed and remain inside the aneurysm.”
Neuroform Atlas was cleared for marketing under a humanitarian device exemption (HDE), a designation awarded to devices used to diagnose and treat conditions that affect fewer than 8,000 people throughout the United States each year. An estimated 6 million people nationwide have an unruptured brain aneurysm of which roughly 25,000 are treated with endovascular or surgical treatments each year. Wide neck aneurysms account for less than 10 percent of treated unruptured aneurysms.
The system is a small nitinol stent that is used alongside metal coils to pack aneurysms in the brain. The stent, positioned through a small tube, is delivered across the neck of the aneurysm to support and pack it.
The hybrid cell stent design provides improvement to wall apposition, ease of use, deployment accuracy and catheter re-entry in the most challenging cases. Deployment accuracy is high, due to its low foreshortening.
Neuroform Atlas is also compatible with the lowest profile coiling microcatheters on the market, enabling physicians to reach smaller, more distal vessels within the neurovasculature, which may not be accessible with larger-bore catheters that are required to deliver other treatment devices.
“This can potentially reduce the number of steps and equipment in each procedure by allowing the physician to use the same microcathether to deliver the Neuroform Atlas and the embolic coils,” said Paul.
Stryker completed enrollment in the Neuroform Atlas US IDE clinical trial for the anterior arm earlier this year. Enrollment continues for the posterior arm. The trial consists of the largest data set on adjunctive stent use with intracranial aneurysm coiling.
The system is currently on sale in 47 countries, including parts of Europe, Asia, and South America.
You Must Be Logged In To Post A CommentRegistroRegistrarse es Gratis y Fácil. Disfruta de los beneficios del Mercado de Equipos Médicos Nuevos y Usados líder en el mundo. ¡Regístrate ahora! |
|