por
Lauren Dubinsky, Senior Reporter | September 14, 2017
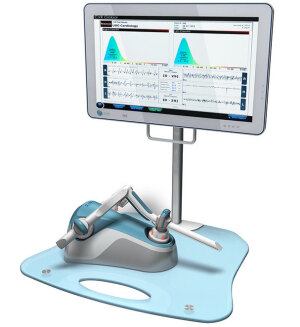
CVR Medical's
Carotid Stenotic Scan device
CVR Medical’s Carotid Stenotic Scan device is showing promise for replacing CT imaging as a cheaper tool for detecting stroke.
Late last week, a research team from Thomas Jefferson University released data from its clinical trials that confirmed the CSS device’s value and efficiency.
“From a clinical standpoint … the most important point is likely that the CSS, in a statistical sense, is very specific,” Phillip J. Bendick, Ph.D., stated. “Nearly all of the tested patients that had carotid artery stenosis were identified without an ‘overread,’ an important measure which allows doctors to recommend steps for further care with confidence.”



Ad Statistics
Times Displayed: 14289
Times Visited: 25 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
Blood flows through the carotid arteries and generates wave patterns that are shaped and altered by any irregularities on the inner artery walls. The CSS device captures the wave patterns and evaluates them with patented algorithms in order to determine a patient’s risk of stroke.
Over 795,000 Americans suffer a stroke every year and about 140,000 of those cases are fatal, according to the Centers for Disease Control and Prevention. In addition, the CDC Foundation reported that stroke and heart diseases cost the U.S. almost $1 billion per day in medical costs and lost productivity.
The cost associated with diagnosing stroke is also high, with the price of CT systems reaching up to $2.5 million and exams ranging from $825 to $4,800, according to New Choice Health.
The CSS device has the potential to cut those costs, since each unit is targeted to cost about $49,000. It also doesn’t require a certified technician to operate it and an exam can be completed in a few minutes.
CVR Medical is currently entering the clinical phase of the development process. The final market version of the CSS device is being assembled and prepared for pivotal trials, which will serve as the basis for FDA clearance.
At the end of August, CVR Medical signed a letter of intent with Canon Virginia Inc., a domestic manufacturing subsidiary of Canon U.S.A. Inc. Once the device receives regulatory approval, Canon will start manufacturing it at its facilities.
CVR Medical is also involved in other manufacturing deals, including a partnership with ADCO Circuit that was announced in March. The company will be the exclusive provider of CSS custom circuit boards for its sensors.
“Internally, in discussions with our engineering and scientific development teams, indications are that we expect the device to improve markedly above the 85% sensitivity level,” Peter Bakema, CEO of CVR Medical, said in a statement. “This, coupled with the expansion of trials for a larger sample size, continues to put us in position for a successful submission to the FDA.”
Back to HCB News

