por
Lynn Shapiro, Writer | January 14, 2009
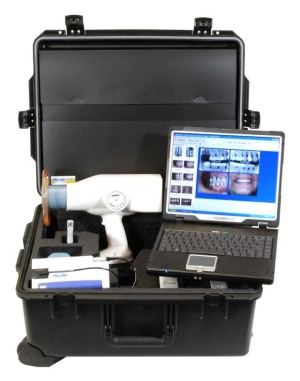
Makers of hand-held X-rays must provide adequate safety procedures customized to the features of the smaller-sized equipment, FDA said this week.
Because smaller devices require the operator and the patient to be closer to the devices, manufacturers must consider how leakage radiation transmitted through the equipment might increase potential X-ray exposure, according to new agency guidelines.
FDA noted that federal performance standards do not address protection for hand-held X-ray devices. FDA suggests makers of these products develop their own precautions and radiation safety procedure instructions.



Ad Statistics
Times Displayed: 13006
Times Visited: 358 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
For example, precautions might include wearing gloves or lead-lined gowns or other protective equipment. An equipment stand and remote switch might also provide protection against radiation exposure.
FDA also advises manufacturers to report exposure measures near the unit, especially where the operator will be located during the procedure. Information on the level of exposure at the handgrips and control devices is particularly useful, the agency says.
For more information, FDA recommends manufacturers refer to their state's radiation control program regulation. "Radiation Safety Consideration for X-ray Equipment Designed for Hand-Held Use", can be viewed at www.fda.gov/cdrh/ocer/guidance/1680.html.