por
John R. Fischer, Senior Reporter | May 24, 2019
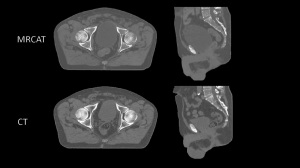
Philips' MRCAT pelvis can now be
used to create treatment plans for
cancers of the pelvis
The FDA has given Royal Philips the okay for its MR-only radiotherapy simulator, MRCAT pelvis (Magnetic Resonance for Calculating Attenuation), in the construction of treatment plans for cancer of the pelvis.
Already approved for planning in cases of prostate cancer, the extension of the solution into the pelvic region of the body is expected to provide clinicians with accurate radiotherapy treatment plans for cancers of the bladder, rectum, anus and cervix. It is one of many regions of the body where the Netherlands-based healthcare giant plans to eventually help create guidance for combating malignancies.
“An exciting potential area for the application of MR-only simulation is the brain, and this is something we're actively working on,” Ardie Ermers, general manager of radiation oncology at Philips, told HCB News. “By expanding the range of cancers for which the treatment planning can take place using MR-only simulation, we're making it more efficient and cost-effective for healthcare providers to deploy MR-only workflows.”



Ad Statistics
Times Displayed: 14290
Times Visited: 25 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
Most patients require a quick CT scan for recording tumor location and tissue density, factors which are important in determining the right radiation dose. They then must undergo MR scans for mapping the tumor and surrounding healthy tissue. Together, both scans can be time-consuming, expensive and create a complex workflow for clinicians, as well as form longer waiting gaps for the start of treatment.
Backed by studies, MRCAT is capable of constructing both soft tissue and density maps from one MR exam that are as accurate as plans based on CT. This eliminates the need for a CT scan, creates a simplified MR-only workflow that is cost-effective for providers, and brings the benefits of MR-only radiation therapy to a broader patient base.
MRCAT pelvis is available on the Philips wide bore digital Ingenia MR-RT platform, which made its debut in 2018 at the ASTRO Annual Meeting in San Antonio, Texas. https://www.dotmed.com/news/story/44953
The simulator, which was first released for prostate cancer radiotherapy planning in 2015, is used in routine clinical settings at several hospitals worldwide and has been used in the treatment planning for over 1000 patients.
“We’ve successfully implemented MR-only simulation in our clinical protocols for pelvic cancer patients,” said Professor Dr. Heikki Minn, of the radiation therapy department at Turku University Hospital in Finland, in a statement. “As a result, we’ve been able to improve efficiency, reduce dosimetric errors introduced by CT-MR registration, and save costs by decreasing redundant imaging.”
MRCAT pelvis is cleared for use by Health Canada and CE marked in Europe.

