Smartphone app de la radiología de diagnóstico de los claros del FDA primer
por
Brendon Nafziger, DOTmed News Associate Editor | February 04, 2011
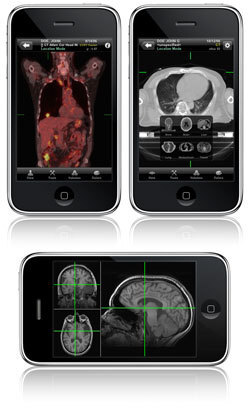
The U.S. Food and Drug Administration has reversed course on one of the first radiology apps ever pulled from the Apple Store.
Now, U.S. radiologists who can't make it to a workstation but need to review medical images can make a diagnosis off their iPhone or iPad.
The FDA said Friday it cleared the first smartphone app that can be marketed for diagnosis, the Mobile MIM reader, made by Cleveland-based MIM Software Inc.
The product is cleared for MRI, CT and nuclear medicine readings for doctors who don't have access to a full workstation, the FDA said. The agency cautioned that the product is not meant to replace the use of workstations.
"This important mobile technology provides physicians with the ability to immediately view images and make diagnoses without having to be back at the workstation or wait for film," said Dr. William Maisel, deputy director for science with the FDA's Center for Devices and Radiological Health, in a statement.
While other products exist on the market letting radiologists review images on their smartphones or tablets, they are marketed as workflow solutions -- they do not have FDA approval to be used for diagnoses.
In fact, almost three years ago, the FDA pulled the Mobile MIM reader off Apple's virtual shelves, arguing it was an unapproved device.
The agency then rejected MIM Software's first two 510(k) applications, saying it was not substantially equivalent to other products on the market, as required under 510(k) guidelines, before granting clearance on Friday.
The FDA said its review of performance tests with the product helped ease their doubts. Tests of the luminance and resolution with radiologists showed MIM Software's product was "sufficient for diagnostic image interpretation under the recommended lighting conditions," the agency said.
The Mobile MIM also includes an interactive contrast test doctors can use to make sure lighting conditions aren't interfering with their ability to read the screen: part of the screen has a slightly different shade than the rest. If doctors see it and can tap on it, it means their lighting is OK, the agency said.
"Establishing a diagnostic protocol for medical imaging is no simple matter for a device like the iPhone or iPad," MIM Software's CTO Mark Cain said, on a note posted on the company's website. "It is critical to understand the characteristics of the device and to establish methods and tools that are safe and effective, while working within those constraints. There has been a gap in the market for a remote imaging device like this, and now it can be filled."
The Mobile MIM reader can be bought from the Apple Store, likely starting next week, the company said. It is also available throughout most of Europe and Central America, as well in India, Australia, Japan, Hong Kong, New Zealand, the Philippines, Singapore, Saudia Arabia and the U.A.E.
