por
Brendon Nafziger, DOTmed News Associate Editor | October 12, 2010
GE Healthcare said Monday its next-generation hybrid SPECT/CT was cleared by the U.S. Food and Drug Administration and is headed to U.S. shores.
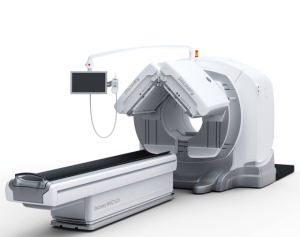
At the European Society of Nuclear Medicine conference in Vienna, GE said it got 510(k) clearance for its Discovery NM/CT 670. The machine combines its BrightSpeed Elite 16-slice CT with a newly built SPECT gantry, the company said.
GE released the first SPECT/CT hybrid in 1999.



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
In its marketing material, the Waukesha, Wisc.-based company said the newer, speedier technology lets it cut traditional bone imaging scan times by half.
GE spokeswoman Rebecca Hayne told DOTmed News four units of the device are scheduled for installation in the United States. To date, more than 25 have been ordered around the world, she said.
Although GE announced the clearance this week, the unit was actually cleared by the FDA in December, Hayne said. It launched in Europe last year.