Advertencia del FDA en los fuegos médicos del equipo
por
Brendon Nafziger, DOTmed News Associate Editor | October 21, 2009
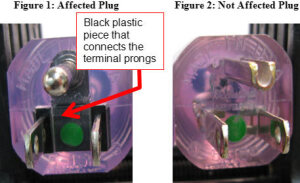
FDA photo comparing
(left) the affected plug
with its black plastic bridge
to the unaffected plug (right)
The FDA released a warning October 19th that possibly defective plugs could cause fires, but the company making the plugs believes it's probably a result of the rough-and-tumble nature of hospitals.
The warning follows voluntary recalls issued by Hospira on August 14 and Abbott on September 4 after the companies received at least 122 reports of sparking, charred bits and fires from machines using plugs the FDA says were made by the Electri-cord Manufacturing Company, based in Westfield, PA.
The cords affected have a black plastic bridge, named a Taller bridge after its original German manufacturer, between the blades. The FDA says if the bridge is burnt, bent or cracked, it should be replaced as soon as possible without jeopardizing patient safety or interrupting treatment.
Cord-maker responds
Electri-cord, which sources the plastic bridges for some of their plugs, stands by their equipment.
"We don't consider it a product defect, we don't consider it a manufacturing defect," Dennis MacDonald, general manager of Electri-cord, tells DOTmed News. "We consider it abuse in the environment these are used in."
MacDonald notes that his company receives weekly U/L inspections -- from the agency that governs quality control in his industry -- and his company has never had process or factory problems. "The U/L reviews all our test records every week, and sends our cords back to U/L labs for more testing. We've never had an issue," he says. And he mentions that Abbott and Hospira have been consulting with his company to find out what's going wrong.
MacDonald says his company has sold over two million of the cords since 2003, and have only had reports of around 26 defects. "That's 13 parts per million defects," he says, noting that anything below 60 per million is considered world class.
MacDonald believes that though the cords meet stringent U/L requirements for hospital use, hospitals are inherently brutal places: when hospital beds are raced from room to room, cords are hurriedly unplugged and the frequent movement of heavy equipment can crush or deform even the most exactingly crafted blades on the plug.
"Blades in hospital cords are solid brass. I mean solid. We get cords where those brass blades are bent at a 90-degree angle. It takes hundreds of pounds of force to do that. It's uncanny to see the condition of some of the cords we get back," he says. "You'd think they were run over by a steam roller."
When asked how hospitals could avoid cord damage, MacDonald recommends doing a "root cause analysis," and he points to Europe, where several years ago regulators suggested moving outlets or receptacles up about four feet higher on the wall. That way, "when the bed is shoved against the wall, the plug isn't behind it," MacDonald says.
