El dispositivo MRI-Dirigido del ultrasonido calienta encima de cáncer de la próstata
por
Brendon Nafziger, DOTmed News Associate Editor | October 12, 2009
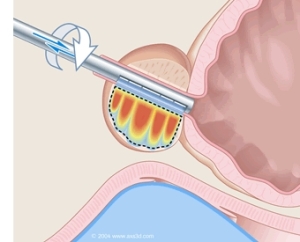
A diagram of Profound's MRI-
guided ultrasound device
Results are on their way from the first human studies in Canada on a device relying on MRI guidance to deliver ultrasound-produced heat to the prostate to fight cancer.
Researchers at the Sunnybrook Health Science Centre in Toronto expect to have preliminary findings soon on a system that might offer a convincing, non-invasive alternative to eliminating cancerous prostates without surgery.
Paul Chipperton, the CEO of Profound Medical, Inc., the Toronto-based company that owns the intellectual property of the device, and which was formed in part by scientists from Sunnybrook, says he anticipates the study, which began in the second quarter of this year, to have enrolled at least 5 to 7 patients before it winds to a close.
How it works
The device uses an MRI to precisely guide a slim, fully MRI-compatible ultrasound wand up the urethra and into the prostate. Once there, the wand emits an acoustic beam that heats up the prostate to a minimum 56 degrees Celsius. At this temperature, biological material becomes denatured. The cancer, and the prostate with it, breaks down and, presumably, will be harmlessly reabsorbed by the body.
The hope is that this method will compare favorably with the current gold standard of prostate cancer treatment, the complete surgical removal of the prostate, but without its side effects.
"[Current] treatments have collateral damage," Chipperton says. In fact, in many men, surgical removal of a prostate can damage the nerves upon which erectile function depends, leaving patients impotent. Other common side effects include urinary incontinence or rectal bleeding -- all contraindications that this new therapy might help men avoid.
If the MRI-ultrasound therapy works as planned, it should also be the fastest treatment out there. Based on animal and mathematical models, Profound believes it could treat a typical prostate -- about 40cc in volume -- in around half an hour, whereas a typical radical prostatectomy can last three hours.
Reports on another experimental treatment, high-intensity focused ultrasound (HIFU), which uses rectally-inserted ultrasounds to ablate the prostate, suggest it also lasts several hours.
On the horizon
Any clinical use for Profound's device is still at least some years away. The proof-of-concept studies -- incidentally, not financed nor conducted by Profound -- that will finish shortly were undertaken to show that the device works safely and can accurately treat diseased tissue in humans, in the same fashion it has previously done in animals.
While Chipperton can't say when he expects full clinical trials to start, he does mention that his company has selected two sites in the U.S. for FDA trials, and expects to begin testing in 2010, if everything goes as planned.
The clinical-grade device, however, which Profound is making, should be finalized before that. "The first instrument will be ready for testing in December, before clinical trials [begin]," Chipperton says.
