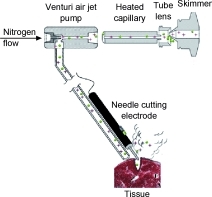
Diagram of the REIMS device.
A device based on mass spectrometry could help surgeons identify cancerous tissue on the fly, according to a study published in Angewandte Chemie (German Chemical Society) last month.
This technique, developed by a team of Hungarian and German scientists, could help surgeons know when they've fully cut out a tumor in under a second.
In current practice, after removing a tumor, surgeons will send a bit of the presumably healthy surrounding tissue for histological analysis to determine if all the cancer was removed -- all while the patient lies anesthetized on the table.



Ad Statistics
Times Displayed: 16169
Times Visited: 33 Final days to save an extra 10% on Imaging, Ultrasound, and Biomed parts web prices.* Unlimited use now through September 30 with code AANIV10 (*certain restrictions apply)
"The good old method is to send it to the pathological lab, wait 30 or 40 minutes, while the patient is lying there under anesthesia wide open, and wait for the result," Zoltan Takats, Ph.D., a chemist at the Justus Liebeg University in Giessen, Germany and lead author of the study, tells DOTmed News.
If results are positive, the surgeons have to cut out more tissue, and do the process over again.
"In some cases, but it's still exotic, they use intraoperative ultrasound, but it's not really precise," Dr. Takats says.
So Dr. Takats and his colleagues developed an alternative that would combine the precision of histological analysis with the speed of an imaging modality.
How REIMS works
Electroscalpels are types of scalpels that use electric shocks to cauterize blood vessels during surgery. They also happen to vaporize small amounts of tissue, sending up clouds of ions.
Dr. Takats and his team realized that, because tumors have unique ion signatures -- made up of roughly 100 to 150 ions -- these could be used to tell cancerous from healthy tissue.
The identifying device developed by Dr. Takats and his colleagues uses a type of mass spectrometry that they call rapid evaporative ionization mass spectrometry, or REIMS. It sucks up ions from tumors burned off during the surgery and feeds them through a program that runs them against a tumor database, giving the surgeon the results in about 800 milliseconds.
With a good database to compare samples against, the device can do more than just recognize cancer: it can grade the tumor, identifying what stage it's in. It can even work on necrotic tissue, making it potentially useful for autopsies.
Dr. Takats says the accuracy rate is around 98 percent. "It is very good," he says. "Even if you look at histology, the response is never any better than 98 percent."
And it can work with more than just electroscalpels.
"We realized actually that any number of surgical methods -- laser surgery, ultrasound-surgery, water-jet surgery -- they all produce ions," he says. "The surgeon doesn't have to do anything [extra], just use the appropriate ion guide."
Currently, much of the work has been done in cultures and on mice. Dr. Takats expects a large-scale study done on dogs with spontaneous (or naturally arising) tumors to be complete in the next nine months.
Commercialization potentials
Dr. Takats and his team are working on what they say is a bigger roadblock than the clinical tests: the cost of mass spectrometry devices, which run from $150,000 to over $1 million.
"The reason behind [this high price] is only the business model of the mass spectrometry companies. The manufacturing costs $20,000 to $50,000. But they are pursuing the business of making only a few instruments for a high price."
The solution that his team hit upon was to develop their own REIMS machine, which he expects to go for around $20,000 or $30,000.
And once the technology is fully proven in clinical trials, he thinks it will be "sold to big players on the market," he says.

