VisionCare's pea-sized
telescope offers hopes
to patients with
a kind of age-related
blindness. (Image courtesy
ViscionCare.)
telescope offers hopes
to patients with
a kind of age-related
blindness. (Image courtesy
ViscionCare.)
Implantable telescope gets FDA OK
July 07, 2010
by Brendon Nafziger, DOTmed News Associate Editor
An implantable miniature telescope designed for patients suffering an age-related "blind spot" received U.S. Food and Drug Administration approval Tuesday.
The pea-sized device, manufactured by VisionCare Ophthalmic Technologies Inc., is surgically implanted in one eye and provides a double magnification of images, according to the FDA.
"This innovation has the potential to provide many people with an improved quality of life," Dr. Jeffrey Shuren, director of the FDA's Center for Devices and Radiological Health, said in a statement.
The product, about 4 mm in diameter and 4 mm long, is designed to help partly restore sight for patients with end-stage age-related macular degeneration.
This disease results in the destruction of the macula, the center of the retina, and can cause an obliterative blurriness in the center of the field of vision.
Age-related macular degeneration is the leading cause of blindness in Americans over age 60, according to the National Eye Institute, with nearly one-third of Americans over 75 at risk for the disease.
The miniature telescope is designed for patients over 75 who are legally blind, with stable, but severe, visual impairment caused by blind spots. The tiny telescope is implanted in one eye to improve central vision, with the other left undisturbed for peripheral vision, the FDA said.
The telescope, invented by VisionCare's founders Yossi Gross and Isaac Lipshitz, comes in two models, one which magnifies images 2.2 times and another 2.7 times. It works for patients with either "wet" or "dry" age-related macular degeneration, VisionCare said in a statement. The "wet" form of the disease is caused by abnormal blood vessels growing underneath the macula, while the "dry" form is caused by the gradual death of macular cells, according to the National Eye Institute.
In order to be eligible to get the device, patients must undergo training with an external (non-implanted) telescope to ensure their vision would improve with the implant and to test whether the eye not getting the device has adequate peripheral vision, the FDA said.
Approval of the device comes in part from the results of a 219-patient, multi-center clinical study, the FDA said. In the study, 90 percent of patients saw some improvement, while three-quarters transitioned from profound or severe visual impairment to moderate impairment, the FDA said.
Nonetheless, the device, because of its relatively large size, does carry some risks, according to the FDA. The FDA said implantation can lead to the loss of corneal endothelial cells , which could, in especially bad cases, require corneal transplants. About five patients in the study needed transplants, the FDA said, with a calculated five-year risk of about 4 percent.
To meet one of the FDA's conditions for approval, Saratoga, Calif.-based VisionCare has agreed to conduct two post-approval studies, the FDA said, following hundreds of patients for two to five years after implantation to better gauge safety and efficacy.
The pea-sized device, manufactured by VisionCare Ophthalmic Technologies Inc., is surgically implanted in one eye and provides a double magnification of images, according to the FDA.
"This innovation has the potential to provide many people with an improved quality of life," Dr. Jeffrey Shuren, director of the FDA's Center for Devices and Radiological Health, said in a statement.
The product, about 4 mm in diameter and 4 mm long, is designed to help partly restore sight for patients with end-stage age-related macular degeneration.
This disease results in the destruction of the macula, the center of the retina, and can cause an obliterative blurriness in the center of the field of vision.
What someone with
age-related macular
degeneration sees. (Image
courtesy National Eye Institute,
National Institutes of Health.)
age-related macular
degeneration sees. (Image
courtesy National Eye Institute,
National Institutes of Health.)
Age-related macular degeneration is the leading cause of blindness in Americans over age 60, according to the National Eye Institute, with nearly one-third of Americans over 75 at risk for the disease.
The miniature telescope is designed for patients over 75 who are legally blind, with stable, but severe, visual impairment caused by blind spots. The tiny telescope is implanted in one eye to improve central vision, with the other left undisturbed for peripheral vision, the FDA said.
The telescope, invented by VisionCare's founders Yossi Gross and Isaac Lipshitz, comes in two models, one which magnifies images 2.2 times and another 2.7 times. It works for patients with either "wet" or "dry" age-related macular degeneration, VisionCare said in a statement. The "wet" form of the disease is caused by abnormal blood vessels growing underneath the macula, while the "dry" form is caused by the gradual death of macular cells, according to the National Eye Institute.
In order to be eligible to get the device, patients must undergo training with an external (non-implanted) telescope to ensure their vision would improve with the implant and to test whether the eye not getting the device has adequate peripheral vision, the FDA said.
Approval of the device comes in part from the results of a 219-patient, multi-center clinical study, the FDA said. In the study, 90 percent of patients saw some improvement, while three-quarters transitioned from profound or severe visual impairment to moderate impairment, the FDA said.
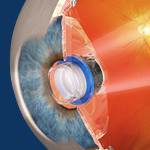
The implantation procedure involves
removing the eye's natural lens
and replacing it with the
tiny telescope implant.
removing the eye's natural lens
and replacing it with the
tiny telescope implant.
Nonetheless, the device, because of its relatively large size, does carry some risks, according to the FDA. The FDA said implantation can lead to the loss of corneal endothelial cells , which could, in especially bad cases, require corneal transplants. About five patients in the study needed transplants, the FDA said, with a calculated five-year risk of about 4 percent.
To meet one of the FDA's conditions for approval, Saratoga, Calif.-based VisionCare has agreed to conduct two post-approval studies, the FDA said, following hundreds of patients for two to five years after implantation to better gauge safety and efficacy.