by
John R. Fischer, Senior Reporter | December 11, 2023
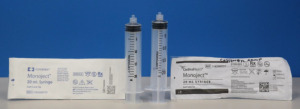
Covidien Monoject syringes (left) and Cardinal Health Monoject syringes (right) (Photo courtesy of FDA)
Following Cardinal Health’s Class I recall of its syringes last month, Becton Dickinson has issued one of its own for its Alaris Infusion Pumps, which are now incompatible with the needles due to design changes made by Cardinal Health as part of a product rebranding.
In 2017, Cardinal Health acquired Covidien Monoject syringes in a $6.1 billion acquisition of select portfolios under Covidien, a subsidiary of Medtronic. Earlier this year in June, the company rebranded them as "Cardinal Health Monoject” syringes, and with the name change, altered their dimensions.
The rebranded solutions have not been validated for use with Alaris Infusion Pumps, which uses PCA Modules programmed to work with the previous sizes. Because of the dimension changes, the pumps either refused to operate or inaccurately estimated the liquid volume within the syringes, leading to over and under infusions and delays in therapy.



Ad Statistics
Times Displayed: 365800
Times Visited: 7089 Quality remanufactured Certified Centrifuges at Great prices! Fully warranted and backed by a company you can trust! Call or click for a free quote today! www.Centrifugestore.com 800-457-7576
According to Reuters, Cardinal Health recalled the syringes in November after reports emerged of incompatibility with patient-controlled pain management pumps and syringe pumps.
In the case of the Alaris Infusion pumps, the FDA has reported 13 injuries so far, but no deaths.
“Use of incompatible syringe sizes and models with the BD Alaris Syringe and PCA Modules can impact syringe pump operation resulting in inaccurate fluid delivery, delayed generation of occlusion alarms, and other potential problems,” said the agency in its recall warning.
A class I recall is the most serious type, issued when a product risks serious injuries or death to patients.
BD previously warned customers, back in September, to stop using the Cardinal Health branded Monoject syringes with the BD Alaris Pump and PCA Modules, issuing an Urgent Medical Device Labeling Correction notice to all customers. In it, the company included updated BD Alaris Syringe and PCA Module Compatibility Lists and customer response forms for providers to fill out and return as confirmation they had received the notification.
While the FDA warns providers not to use Cardinal Health's Monoject syringes, it says that the original Covidien-sized ones are acceptable.
Covidien and Medtronic recently faced a recall of their own in July 2022 for nine different analysis catheters after an issue in more than one million
led to leaking. This included several models of their Palindrome Chronic catheters and one model of the Mahurkar Chronic Carbothane catheter.
The year before, Medtronic
recalled its HeartWare ventricular assist device (HVAD), for hemodynamic support, due to reports of adverse neurological incidents, including stroke and deaths.

