by
John R. Fischer, Senior Reporter | October 17, 2019
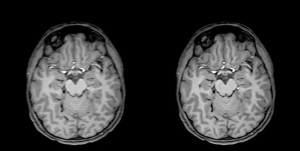
SubtleMR denoises and enhances the resolution
of MR images
The FDA has greenlighted Subtle Medical to commence sales of its image processing software, SubtleMR, to providers throughout the U.S.
The AI-powered solution is designed to denoise and enhance the resolution of MR scans for better quality to ensure faster and more accurate diagnoses.
"One of the most exciting things about deep learning reconstruction is how it redefines the usual negotiation between exam time and image quality. This could lead to significant downstream value for imaging operations and for patient experience," said Christopher Hess, chair of the department of radiology and biomedical imaging at the University of California, San Francisco, in a statement.



Ad Statistics
Times Displayed: 45718
Times Visited: 1370 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
MR scan interpretations can be challenging to conduct for images of patients who have difficulty holding still for long periods of time, or if multiple artifacts are present. Such issues can impair the quality of images and may lead to the need for re-scans.
Compatible with any manufactured MR scanner and PACS system, SubtleMR seamlessly integrates within radiology workflows by deploying proprietary deep learning algorithms to enhance images.
"We are pleased to receive FDA clearance for SubtleMR, and we look forward to helping radiology departments and imaging centers get the most out of their existing MR scanners," said Enhao Gong, founder and CEO of Subtle Medical, in a statement. "This is an important milestone for the company as it broadens our portfolio of technologies developed for radiologists and their patients."
The approval comes nearly a year after the FDA
gave the okay for Subtle Medical’s other medical image enhancement software, SubtlePET, which improves images generated from up to four-times-faster PET scans. A third product, SubtleGAD, is currently under development and will help reduce gadolinium dosage during contrast-enhanced MR exams. The NIH recently offered the company a $1.6 million grant to fund research on the technology.
SubtleMR is currently in pilot clinical use at multiple university hospitals and imaging centers.

