by
John W. Mitchell, Senior Correspondent | July 12, 2018
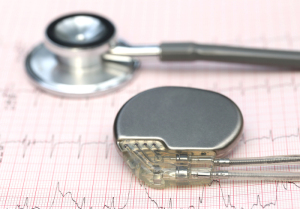
Patients with implantable cardiac devices, such as pacemakers, can safely receive MR scans, as long as radiologists and electrophysiologists work together under an exacting protocol.
That’s the conclusion of an article recently published in
Radiology, authored by experts at the Hospital of the University of Pennsylvania and Johns Hopkins University.
“An increasing number of patients are denied valuable diagnostic information from MR examinations because they have implantable defibrillators or pacemakers,” Dr. Timothy Markman, Cardiology Fellow at the University of Pennsylvania and co-author told HCB News. “We wanted to clarify whether MR examinations could be safely performed in these patients.”



Ad Statistics
Times Displayed: 172944
Times Visited: 3140 For those who need to move fast and expand clinical capabilities -- and would love new equipment -- the uCT 550 Advance offers a new fully configured 80-slice CT in up to 2 weeks with routine maintenance and parts and Software Upgrades for Life™ included.
The team reviewed recent data and findings on the procedure. This included a study of 1,509 patients with cardiac implants published in
The New England Journal of Medicine in December that concluded the procedure did not cause any “long-term clinically significant events.” Markman and his colleagues discussed what protocols radiologists, electrophysiologists, and nurse specialists need to follow to deliver MR diagnostics safely.
“It is now clear that MR examinations can be safely performed on patients with implantable cardiac devices when standardized programming and monitoring are used,” said Markman.
However, it is "essential" that radiologists consult with electrophysiologists familiar with the necessary device reprogramming. The patient must also be monitored during the MR scan by either an electrophysiologist or a specially trained nurse, to ensure they are tolerating the exam well.
The study found that while the FDA has approved new “MR-conditional” pacemaker designs, there are many patients fitted with older “legacy” devices. Past concerns have been that under the powerful magnetic atmosphere in an MR, the device might move, the leads could heat up, the device might generate the wrong rhythm and other possible complications. In the article, the authors discuss such factors as magnet strength and the necessary device reprogramming indicated for safe MR use on such patients.
The issue of MR scans for patients with an implantable cardiac device has been gaining momentum in recent years. MR, more and more, is becoming the imaging modality of choice because of its superiority in soft tissue diagnosis. The study team believes that because MR technology keeps improving, past restrictions should be revisited.
“We hope that improved awareness of these results will lead to more radiologists and electrophysiologists developing protocols for the safe performance of MR examinations on these patients,” Markman said.