FDA permits EBR Systems to initiate study for wireless cardiac pacing system
by Lauren Dubinsky, Senior Reporter | September 15, 2016
Cardiology
Medical Devices
Population Health
A step toward FDA approval
About 30 percent of patients who receive conventional cardiac resynchronization therapy (CRT) don't respond to it — but the world's smallest, wireless implantable device for cardiac pacing may help solve that.
The FDA has granted an Investigational Device Exemption for EBR Systems Inc.'s Wireless Stimulation Endocardially (WiSE) Technology for CRT. This will allow the developer to initiate a large study in the U.S. to demonstrate the safety and effectiveness of WiSE in hopes of receiving FDA approval.
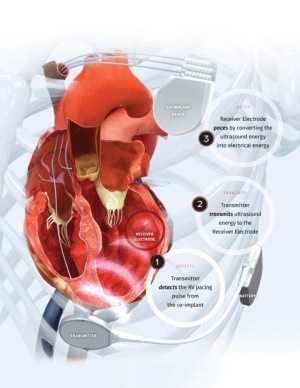
A tiny electrode is placed in the left ventricle and with every heartbeat, it receives a synchronized ultrasound signal from a small transmitter between the patient's ribs. The sound waves are converted into electrical energy, which provides cardiac pacing.
The WiSE technology eliminates the need for a left ventricular lead and allows the physician to position the stimulation point at the ideal location. Internal stimulation is thought to be similar to the natural activation pattern of the heart, compared to the current surface pacing techniques.
Studies have shown that successful CRT can reduce heart failure symptoms, hospitalization and mortality because it synchronizes both the left and right ventricles.
Wire leads and large implants that are placed inside of the left ventricle can lead to clots, heart attacks or stroke. The wire leads can also break or fail, which causes complications in about 12 percent of cases.
The WiSE cardiac implant is about the size of a large grain of rice and it doesn't require a pacing wire on the outside of the heart's left ventricle.
It's estimated that over $1 billion of the $3.5 billion spent per year on CRT devices provides no benefit to the patient, but WiSE can potentially benefit 1.5 million patients around the world.
The Stimulation of the Left Ventricle Endocardially — CRT study (SOLVE-CRT) will enroll 350 patients that have either failed conventional CRT or were previously untreatable with conventional CRT systems. EBR Systems is expecting to initiate the study in mid-2017.
The company conducted a previous study called SELECT-LV and the results were presented in May by Dr. Vivek Reddy, cardiologist and professor at Mount Sinai Hospital, at the Heart Rhythm Society Scientific Sessions.
For that study, 97 percent of 35 patients who had failed conventional CRT therapy were implanted successfully with WiSE Technology. Out of those patients, 85 percent experienced an improvement in symptoms at the six-month point.
EBR Systems is planning on introducing the second generation of the WiSE Technology in Europe in early 2017 as well as include it in the SOLVE-CRT study. The transmitter size has been reduced by half and the battery life is expected to be the same as conventional systems.
The FDA has granted an Investigational Device Exemption for EBR Systems Inc.'s Wireless Stimulation Endocardially (WiSE) Technology for CRT. This will allow the developer to initiate a large study in the U.S. to demonstrate the safety and effectiveness of WiSE in hopes of receiving FDA approval.
A tiny electrode is placed in the left ventricle and with every heartbeat, it receives a synchronized ultrasound signal from a small transmitter between the patient's ribs. The sound waves are converted into electrical energy, which provides cardiac pacing.
The WiSE technology eliminates the need for a left ventricular lead and allows the physician to position the stimulation point at the ideal location. Internal stimulation is thought to be similar to the natural activation pattern of the heart, compared to the current surface pacing techniques.
Studies have shown that successful CRT can reduce heart failure symptoms, hospitalization and mortality because it synchronizes both the left and right ventricles.
Wire leads and large implants that are placed inside of the left ventricle can lead to clots, heart attacks or stroke. The wire leads can also break or fail, which causes complications in about 12 percent of cases.
The WiSE cardiac implant is about the size of a large grain of rice and it doesn't require a pacing wire on the outside of the heart's left ventricle.
It's estimated that over $1 billion of the $3.5 billion spent per year on CRT devices provides no benefit to the patient, but WiSE can potentially benefit 1.5 million patients around the world.
The Stimulation of the Left Ventricle Endocardially — CRT study (SOLVE-CRT) will enroll 350 patients that have either failed conventional CRT or were previously untreatable with conventional CRT systems. EBR Systems is expecting to initiate the study in mid-2017.
The company conducted a previous study called SELECT-LV and the results were presented in May by Dr. Vivek Reddy, cardiologist and professor at Mount Sinai Hospital, at the Heart Rhythm Society Scientific Sessions.
For that study, 97 percent of 35 patients who had failed conventional CRT therapy were implanted successfully with WiSE Technology. Out of those patients, 85 percent experienced an improvement in symptoms at the six-month point.
EBR Systems is planning on introducing the second generation of the WiSE Technology in Europe in early 2017 as well as include it in the SOLVE-CRT study. The transmitter size has been reduced by half and the battery life is expected to be the same as conventional systems.
You Must Be Logged In To Post A CommentRegisterRegistration is Free and Easy. Enjoy the benefits of The World's Leading New & Used Medical Equipment Marketplace. Register Now! |
|









