Breast imaging products help with coronavirus backlog
July 27, 2020
by Lisa Chamoff, Contributing Reporter
With the COVID-19 pandemic pushing breast cancer screening to the back burner for many patients, scanner and software manufacturers have more reason than ever to bring products to the market that make scans faster and more comfortable, and helping radiologists manage their growing worklists.
At the same time, breast CT is emerging as a modality for breast cancer diagnosis, providing 3D images with shorter scan times and at a lower cost than breast MR.
Here’s a look at what’s new in the last year from more than a dozen companies.
AB-CT
AB-CT — Advanced Breast-CT GmbH has developed a breast-CT scanner, nu:view, which was released with CE mark clearance in June 2018. The company plans to apply for FDA clearance as well.
“The American market is a big market for us, but we need to undergo a Premarket Approval (PMA) for the FDA clearance” said Ludger Hajduk, head of customer relationship management for AB-CT.
The company has been building awareness of the technology, which provides real 3D breast imaging without compression, at trade shows such as RSNA.
One of nu:view’s key technologies comes from its detector, Hajduk said. The concavely shaped single-photon counting hybrid detector is made of cadmium telluride (CdTe), a highly sensitive material that converts X-rays directly into electrical signals, providing high image quality with low radiation dose.
“Scan times are in the range of seven to 12 seconds, while the gantry rotates around the breast in (a) downwards-oriented spiral trajectory”, Hajduk said.
In Europe, the scanner is in use for diagnostic purposes both with and without contrast media enhancement.
“One of the strengths of nu:view is both the display of microcalcifications and an excellent soft tissue delineation”, Hajduk said.
There are no restrictions regarding age or gender of the patient, and the system is also suitable for patients with small breasts, dense breasts, mastodynia or implants, Hajduk said.
“The compelling reason for women is doing the scan without any compression,” Hajduk said.
Candelis
Last fall, Candelis released the newest version of its Advanced Breast Imaging Enterprise Viewer on the ImageGrid PACS.
The company partners with a number of OEMs to route large mammography and tomosynthesis images, so that radiologists can read them quickly, and the latest release has increased the efficiency of image movement.
"We partner with GE, Hologic and Siemens to help move the large mammo and tomo data sets from acquisition on the gantry to the reading workstation," said Robert Van Uitert, vice president of marketing at Candelis. "When a radiologist needs to review five to ten years of prior patient images, this requires movement of a lot of data per patient."
The system uses what Van Uitert calls a "thin client viewer", rather than a traditional "thick client workstation" which requires sending a new data set to each radiologist workstation, resulting in an increase of data movement and reduced speed.
ImageGrid PACS provides pre-caching of images for quick image review and enhanced mammography prefetch for review of prior patient images.
"Our retrieval of the prior studies (is) during off-hour times so it's not bogging down the network," Van Uitert said. "We're making sure it's on the viewer before the radiologist sits down to read. Often there will be some kind of inconsistencies between the current image and prior studies — a difference in the name, for example — and we have services in background that are able to resolve those differences. Radiologists are going to receive the images that they need to read sooner."
The Advanced Breast Imaging Enterprise Viewer update has also added support for the reading of 3D breast ultrasound exams.
CureMetrix
CureMetrix is currently conducting a large reader study for its investigational cmAssist product, computer-aided detection (CAD) software that uses AI to help the radiologist identify, mark and score regions of interest on screening and diagnostic mammograms.
“While we have the product installed around the world and installed in the U.S., we didn’t want to bring it to market until we had an efficacy that was better than anything else out there,” said Kevin Harris, the president of CureMetrix. “It’s battling 20 years of CAD products that are not great.”
A reader study included 500 biopsy-confirmed cancer cases, going back six years to earliest actionable incidence of cancer. The doctors read each case twice. The first time they did it without cmAssist and noted their findings. They came back a month later and turned on cmAssist.
Using cmAssist improved the performance of the seven radiologists who participated by 27% and they were able to confirm a third more cancers and had no increase in recall rates.
The clinical trial for FDA clearance of the product will have “considerably” more doctors and cases, Harris said. “However, since the previous study was on the most difficult cases we could find, and our performance was outstanding, we fully expect our pivot study to demonstrate an efficacy and thus utility in the market far exceeding any competitive product.”
The goal is to partner cmAssist with CureMetrix’s FDA-cleared cmTriage product to help improve reading time, streamline clinical operations, reduce radiologist burnout and improve cancer detection rates.
“These two products go together hand in glove,” Harris said.
Harris says the cmTriage product — which pre-processes and triages radiologists’ mammography worklists, will be key to process the backlog of mammograms that are not taking place during the COVID-19 pandemic.
"When doors open up again, clinics are going to have a flood of patients," Harris said. "There are 3 million mammograms per month that have been deferred during this crisis and cmTriage will allow them to prioritize those patients and get the most suspicious cases to the front of the list."
Densitas
In February of this year, the company has updated its densitas densityai breast density assessment software, which was first FDA-cleared in 2018, with a deep learning solution. The technology was previously based on handcrafted features and machine learning, and Mo Abdolell, chief executive officer of Densitas, believes the new approach is more aligned with how radiologists visualize breast density in mammograms.
“The algorithm still has the same purpose,” Abdolell said. “It’s still classifying breast density tissue composition according to the ACR BI-RADS 5th Edition density scale, but we use a deep learning approach to build out our algorithm. What it does is learn on its own from the data set without requiring handcrafted features.”
The company has also expanded its densitas qualityai solution, an AI-based evaluation of mammographic image quality with a focus on positioning errors, into a full web-based analytics platform.
The solution is now available both at the point of care and presented in a system-level context, with access to data of all mammograms performed across a health system.
“With AI automation, there’s now a unique capacity to generate deeper insights into system level performance and digitalize reporting workflows,” Abdolell said. “Because you have access to all of this data across a whole health system, you can apply continual quality assurance. If you’re going to deliver quality care, you have to be able to employ embedded analytics to all of your mammograms collectively. You get a bird’s eye view and you get to see what’s going on in your mammography facilities across your health system.”
Fujifilm
Last year, Fujifilm announced that three new image processing and software advancements for its ASPIRE Cristalle digital mammography system – S-View, Iterative Super-resolution Reconstruction (ISR), and Tomosynthesis Spot — had been FDA-cleared.
“The technology allows us to provide a better view in synthetic 2D images with almost 50% less dose, compared to regular 2D plus tomosynthesis,” said Rick Banner, senior director of marketing for FUJIFILM Medical Systems U.S.A. Inc.
In July of 2019, the company also debuted a tomosynthesis biopsy option for the ASPIRE Cristalle, which provides guidance to calculate 3D coordinates of a suspicious lesion in the breast, in addition to the traditional stereotactic method. The option is designed to provide a more efficient workflow that reduces the exam time.
“We want to make sure radiologists are able to provide this option to target lesions that were previously undetectable on tomo biopsy,” Banner said.
GE Healthcare
The novel coronavirus pandemic has also changed how companies provide service and train customers on its equipment. GE Healthcare has been continuously improving capabilities of its OnWatch preventive maintenance software, and has been piloting a virtual online trainer for mammographers.
“Our goal is to help our customers with this tremendous backlog they will be facing,” said Agnes Berzsenyi, president and chief executive officer of women’s health for GE Healthcare.
Last year, GE Healthcare and healthcare improvement company Premier Inc. announced they would be collaborating to develop a model for a same-day breast cancer diagnosis and treatment model, with streamlined screening and same-day biopsies.
“With our Pristina Serena, we can transform a screening room to an interventional suite in a few minutes,” Berzsenyi said.
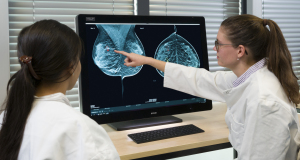
Hologic
Over the last two years, Hologic has made a few acquisitions, including of digital specimen company Faxitron Bioptics, bioresorbable tissue marker developer Focal Therapeutics and ultrasound manufacturer SuperSonic Imagine.
The Supersonic Imagine acquisition in August of last year provided Hologic with an entrée into the ultrasound market using the company’s Aixplorer and MACH 30 platform.
“Our global reach and footprint will create new commercial opportunities with these platforms,” said Samir Parikh, the global vice president of research and development for Hologic.
Last November, the FDA approved Hologic’s 3DQuorum Imaging Technology powered by Genius AI, which works with Hologic’s Clarity HD high resolution imaging technology to reduce tomosynthesis image volume for radiologists by 66%. This results in improved read time.
“You can reduce almost an hour of an eight-hour workday,” Parikh said.
Also last November, the company debuted its Unifi Workspace, which integrates the reading of MR, ultrasound and mammography into one platform, which Parikh said also improved workflow efficiency.
Radiologists also get personalized hanging protocols when they’re logged in.
iCAD
iCAD has continued improving the overall sensitivity and specificity performance of its ProFound AI software, which was FDA cleared in December 2018. The software aids in the detection of breast cancer in digital breast tomosynthesis and assists in clinical decision-making and improving reading efficiency, providing radiologists with Certainty of Finding lesion and Case Scores.
It is also focusing on further enhancing the ProFound AI algorithm by adding the ability to analyze a woman’s prior mammography images to better determine the likelihood of malignancy.
"In other words, ProFound AI will be able to determine if a mass or calcification cluster has changed since the last mammogram, which can be an important indication of cancer," said Michael Klein, chairman and chief executive officer of iCAD.
The software, which the company says provides a 52.7% reduction in reading time for radiologists, will help resolve the issue of prioritizing patients in the wake of the COVID-19 pandemic, Klein said.
In late 2020, iCAD will be launching a short-term risk assessment solution that can help determine a women’s risk of developing cancer prior to her next screening. The solution, ProFound AI Risk, was developed in partnership with researchers at the Karolinska Institute in Stockholm, Sweden.
The risk model assesses the risk of developing breast cancer based on the patient’s age and information found in their mammography images. Along with age, the risk model factors in the presence of masses, calcifications, breast density, and their asymmetry to determine the risk of developing breast cancer in the next one or two years or before the next screening.
A large retrospective study conducted in Sweden showed that the risk model significantly outperforms traditional risk models that are based on family history, lifestyle factors and genetics when determining short-term risk, Klein said.
"We believe this new risk model will be a catalyst in the long-overdue transition from age-based screening to risk-based screening," Klein said. "This product will offer a more holistic clinical approach that can provide clinicians with a broader view of each individual patient’s case and short-term breast cancer risk. ... We believe this AI-centric panoramic view of a woman will lead to even earlier cancer detections, as well as a much more appropriate utilization of breast cancer screening resources including both supplemental screening and screening frequency."
The product will first be available for full-field digital mammography with a primary focus on the EU market in the third quarter of 2020. In early 2021, the company plans to release a version of ProFound AI Risk for digital breast tomosynthesis.
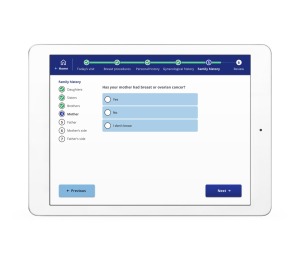
Ikonopedia
Ikonopedia offers a cloud-based structured breast reporting system known as the Ikonopedia Breast Reporting and Tracking software, as well as a tablet-based patient questionnaire that employs a web-based version of the Tyrer-Cuzick Breast Cancer risk assessment tool.
The patient questionnaire was recently updated to include any language — one customer, the Mount Sinai Health System in New York City, uses 14 different languages — which helps to reduce the cost of translation services.
“(Providers have) also seen that the follow-up care has been higher, given that all patients receive their results and reminder letters in their native language,” said Emily Crane, Ikonopedia's chief executive officer.
Ikonopedia also utilizes competing mortality in the Tyrer Cuzick risk assessment that creates the most specific breast cancer risk possible.
“As I age, the other things that could cause my death become higher,” Crane explained. “What it allows them to do and spend their healthcare dollars more wisely.”
As part of its Breast Reporting and Tracking software, Ikonopedia also has a tool that can track images for MQSA-Enhancing Quality Using the Inspection Program (EQUIP) audits, which evaluates image quality.
“We developed an EQUIP tool so they can actually track and audit those images in an automated way,” Crane said. “We also create all reports that are needed in the breast center including MQSA, EQUIP, and timeliness of follow up care.
Koios Medical Inc.
Last July, Koios announced FDA clearance of its Koios DS Breast 2.0, AI-based decision support software that assists radiologists in analyzing and classifying suspicious breast ultrasound images.
The software provides an AI-generated finding, providing a probability of malignancy within the appropriate BI-RADS category and descriptors for reporting.
“When using ultrasound, something is suspicious,” said Chad McClennan, president and chief executive officer of Koios. “Koios DS is an instantaneous second opinion for those cases.”
The software’s ensemble of algorithms have been trained on hundreds of thousands of images from a variety of patient demographic profiles derived from a wide range of ultrasound system manufacturers, and McClennan says customers have seen workflow improvements combined with a reduction in benign biopsies and avoidable, unnecessary follow-up appointments, while catching more cancers earlier.
Koning
As Koning continues to market its Koning Breast CT (KBCT) system, the company recently applied for six new Current Procedural Terminology (CPT) codes and anticipates an announcement from the American Medical Association sometime after press time.
New CPT codes provide the first step toward reimbursement for breast CT in place of a diagnostic mammographic workup, allowing physicians to order exams with or without contrast.
Previous to dedicated codes, facilities were required to use unlisted codes, making reimbursement uncertain, said David Georges, president of the company’s USA division.
Breast CT is also seen as low-cost alternative to MR for breast cancer diagnosis.
“We believe the clinical and payer environment will embrace this technology very quickly,” Georges said.
Medical Scientific
In the second half of 2019, Medical Scientific released a wireless retrofit detector that can be used to upgrade analog mammography systems.
Since the detector is wireless, it is portable and can be moved from one system to another, ideal for a facility with multiple rooms.
“They can effectively upgrade both systems for the cost of one detector,” said Steven Veyland, chief operating officer of Medical Scientific.
The detector is targeted at developing markets in South America, Southeast Asia and Africa, or facilities that have purchased CR systems.
The detector has a 49.5-micron pixel size, versus the 70- to 85-micron pixel size from competitors.
“It provides a much higher image resolution,” Veyland said. “The resulting image they get will be a higher resolution than any 2D system on the market.”
The company also has a mammography reading workstation with a 5 megapixel monitor.
“We provide the complete solution,” Veyland said.
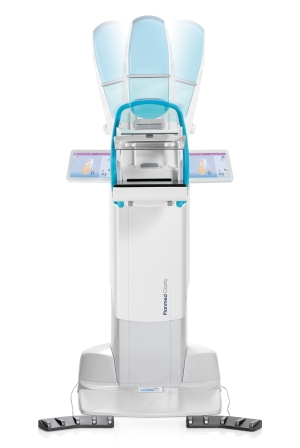
Planmed
The company has improved the algorithm for the synthetic 2D image on its Planmed Clarity 3D digital breast tomosynthesis system
“It looks like a normal 2D image so the radiologist doesn’t have to look at something different,” said Jukka Erkkilä, clinical and product management director of Planmed Oy. “The radiologists really prefer it and sometimes they prefer it over the normal 2D.”
Planmed also had a software update in December that improved post processing for tomosynthesis and stereotactic biopsy.
“It improves tissue separation and also sharpness,” Erkkilä said. “Microcalcifications are clearer.”
The update also provides support for AccuBoost brachytherapy.
Qlarity Imaging
Formerly known as Quantitative Insights, the company was acquired by Paragon Biosciences, a Chicago-based incubator, in June of 2019.
Since then, Qlarity Imaging has made upgrades to the usability and efficiency of its QuantX AI-based decision support software for breast cancer diagnosis using MRI.
The software mimics an app-style workflow, said Robert Tomek, chief technology officer of Qlarity Imaging.
“There are less clicks to get where you need to go,” Tomek said. “We’re putting what the users want right in front of them.”
The company is also researching other breast imaging areas outside of MR, with plans to develop a single platform for all breast imaging modalities in the future.
ScreenPoint Medical
In late 2018, ScreenPoint Medical received FDA clearance for Transpara, its AI application to assist radiologists with reading screening 2D mammograms. In March 2020, ScreenPoint received FDA clearance for use with 3D mammograms — the first AI application to be cleared for use with both 2D and 3D mammograms.
The product, which is distributed in the United States, Australia, New Zealand and parts of Asia by Volpara, provides radiologists with a “second set of eyes,” said David Lee, ScreenPoint Medical’s marketing manager.
The company said customers are also eyeing the solution to help them with the backlog of screening exams due to the COVID-19 pandemic.
Siemens Healthineers
The company has a software update for its MAMMOMAT Revelation that has just received FDA clearance. Called VC20, the software will include efficiency boosts, including faster calibration of the system — from 76 minutes down to five minutes — as well as a faster booting time of four minutes versus 20 minutes and 50-degreeTomoFlow, a feature that permits faster exam times.
With the 50-degree TomoFlow, the patient doesn’t have to wait in between image captures with a compressed breast, providing a better patient experience, said Sammie Stein, women's health product manager at Siemens Healthineers North America.
“Before, the technologist would take the image and wait,” Stein said. “Now, they can take images back to back and the reconstruction happens in the background.”
Storage on the system has also been doubled and image transfer is more flexible, and can be done after each exam or each exposure.
“There is flexibility in what works best for their practice,” Stein says.
The company also plans to release new accessories for biopsies, including a spacer plate that allows for easier imaging of smaller breasts, allowing for less space between the compression panel and the table, and a lateral needle holder for easier lateral targeting.
Siemens Healthineers also released an upgrade to its XA20 software for the BioMatrix family of MR scanners, including the 3T MAGNETOM Vida and Lumina systems and the 1.5T MAGNETOM Sola and Altea scanners.
The update includes an AI workflow enhancement for breast biopsy imaging, with automatic calculation to improve needle positioning together with the Select&GO feature to see target positioning information on the scanner itself.
“Before, you would have to plan and target each lesion individually, moving between the scan room and control room to take each biopsy,” said Kelly Parker, senior product marketing manager for MR at Siemens Healthineers North America. “Now you can plan the targets for all biopsies at the same time, then return to the scan room to perform the biopsies, resulting in a more streamlined workflow for the clinician and the patient.”
Volpara
Last year, Volpara Solutions acquired MRS Systems Inc., a software company with a reporting system that allows healthcare providers to document patient history, risk factors and radiology findings from breast screening exams.
Mark Koeniguer, chief commercial officer and president of the U.S. division of Volpara, said the merger brings together VolparaRisk software, a breast cancer risk assessment software, and Aspen Breast product, marking a shift from traditional pure screening to risk stratified screening.
“With the acquisition of MRS last year … we can fundamentally change how women are triaged to provide supplemental imaging based on risk,” Koeniguer said. “If they are dense but not high-risk, they might be a candidate for ultrasound and not MR. Whereas, a woman at high risk may be a candidate for MR despite other factors. The two (products) fit together and speak to one another.”
Customers of the VolparaPlatform, which was launched at last year’s RSNA, include customers across the imaging spectrum, from small imaging centers to large IDNs, and it has increased breast cancer detection rates.
“A lot of this information existed in various forms in different parts of the department, but it wasn’t easily accessible during the reading of the mammogram,” Koeniguer said. “There are sites using this and their detection rates are higher.”
At the same time, breast CT is emerging as a modality for breast cancer diagnosis, providing 3D images with shorter scan times and at a lower cost than breast MR.
Here’s a look at what’s new in the last year from more than a dozen companies.
AB-CT
AB-CT — Advanced Breast-CT GmbH has developed a breast-CT scanner, nu:view, which was released with CE mark clearance in June 2018. The company plans to apply for FDA clearance as well.
“The American market is a big market for us, but we need to undergo a Premarket Approval (PMA) for the FDA clearance” said Ludger Hajduk, head of customer relationship management for AB-CT.
The company has been building awareness of the technology, which provides real 3D breast imaging without compression, at trade shows such as RSNA.
One of nu:view’s key technologies comes from its detector, Hajduk said. The concavely shaped single-photon counting hybrid detector is made of cadmium telluride (CdTe), a highly sensitive material that converts X-rays directly into electrical signals, providing high image quality with low radiation dose.
“Scan times are in the range of seven to 12 seconds, while the gantry rotates around the breast in (a) downwards-oriented spiral trajectory”, Hajduk said.
In Europe, the scanner is in use for diagnostic purposes both with and without contrast media enhancement.
“One of the strengths of nu:view is both the display of microcalcifications and an excellent soft tissue delineation”, Hajduk said.
There are no restrictions regarding age or gender of the patient, and the system is also suitable for patients with small breasts, dense breasts, mastodynia or implants, Hajduk said.
“The compelling reason for women is doing the scan without any compression,” Hajduk said.
Candelis
Last fall, Candelis released the newest version of its Advanced Breast Imaging Enterprise Viewer on the ImageGrid PACS.
The company partners with a number of OEMs to route large mammography and tomosynthesis images, so that radiologists can read them quickly, and the latest release has increased the efficiency of image movement.
"We partner with GE, Hologic and Siemens to help move the large mammo and tomo data sets from acquisition on the gantry to the reading workstation," said Robert Van Uitert, vice president of marketing at Candelis. "When a radiologist needs to review five to ten years of prior patient images, this requires movement of a lot of data per patient."
The system uses what Van Uitert calls a "thin client viewer", rather than a traditional "thick client workstation" which requires sending a new data set to each radiologist workstation, resulting in an increase of data movement and reduced speed.
ImageGrid PACS provides pre-caching of images for quick image review and enhanced mammography prefetch for review of prior patient images.
"Our retrieval of the prior studies (is) during off-hour times so it's not bogging down the network," Van Uitert said. "We're making sure it's on the viewer before the radiologist sits down to read. Often there will be some kind of inconsistencies between the current image and prior studies — a difference in the name, for example — and we have services in background that are able to resolve those differences. Radiologists are going to receive the images that they need to read sooner."
The Advanced Breast Imaging Enterprise Viewer update has also added support for the reading of 3D breast ultrasound exams.
CureMetrix
CureMetrix is currently conducting a large reader study for its investigational cmAssist product, computer-aided detection (CAD) software that uses AI to help the radiologist identify, mark and score regions of interest on screening and diagnostic mammograms.
“While we have the product installed around the world and installed in the U.S., we didn’t want to bring it to market until we had an efficacy that was better than anything else out there,” said Kevin Harris, the president of CureMetrix. “It’s battling 20 years of CAD products that are not great.”
A reader study included 500 biopsy-confirmed cancer cases, going back six years to earliest actionable incidence of cancer. The doctors read each case twice. The first time they did it without cmAssist and noted their findings. They came back a month later and turned on cmAssist.
Using cmAssist improved the performance of the seven radiologists who participated by 27% and they were able to confirm a third more cancers and had no increase in recall rates.
The clinical trial for FDA clearance of the product will have “considerably” more doctors and cases, Harris said. “However, since the previous study was on the most difficult cases we could find, and our performance was outstanding, we fully expect our pivot study to demonstrate an efficacy and thus utility in the market far exceeding any competitive product.”
The goal is to partner cmAssist with CureMetrix’s FDA-cleared cmTriage product to help improve reading time, streamline clinical operations, reduce radiologist burnout and improve cancer detection rates.
“These two products go together hand in glove,” Harris said.
Harris says the cmTriage product — which pre-processes and triages radiologists’ mammography worklists, will be key to process the backlog of mammograms that are not taking place during the COVID-19 pandemic.
"When doors open up again, clinics are going to have a flood of patients," Harris said. "There are 3 million mammograms per month that have been deferred during this crisis and cmTriage will allow them to prioritize those patients and get the most suspicious cases to the front of the list."
Densitas
In February of this year, the company has updated its densitas densityai breast density assessment software, which was first FDA-cleared in 2018, with a deep learning solution. The technology was previously based on handcrafted features and machine learning, and Mo Abdolell, chief executive officer of Densitas, believes the new approach is more aligned with how radiologists visualize breast density in mammograms.
“The algorithm still has the same purpose,” Abdolell said. “It’s still classifying breast density tissue composition according to the ACR BI-RADS 5th Edition density scale, but we use a deep learning approach to build out our algorithm. What it does is learn on its own from the data set without requiring handcrafted features.”
The company has also expanded its densitas qualityai solution, an AI-based evaluation of mammographic image quality with a focus on positioning errors, into a full web-based analytics platform.
The solution is now available both at the point of care and presented in a system-level context, with access to data of all mammograms performed across a health system.
“With AI automation, there’s now a unique capacity to generate deeper insights into system level performance and digitalize reporting workflows,” Abdolell said. “Because you have access to all of this data across a whole health system, you can apply continual quality assurance. If you’re going to deliver quality care, you have to be able to employ embedded analytics to all of your mammograms collectively. You get a bird’s eye view and you get to see what’s going on in your mammography facilities across your health system.”
Fujifilm
Last year, Fujifilm announced that three new image processing and software advancements for its ASPIRE Cristalle digital mammography system – S-View, Iterative Super-resolution Reconstruction (ISR), and Tomosynthesis Spot — had been FDA-cleared.
“The technology allows us to provide a better view in synthetic 2D images with almost 50% less dose, compared to regular 2D plus tomosynthesis,” said Rick Banner, senior director of marketing for FUJIFILM Medical Systems U.S.A. Inc.
In July of 2019, the company also debuted a tomosynthesis biopsy option for the ASPIRE Cristalle, which provides guidance to calculate 3D coordinates of a suspicious lesion in the breast, in addition to the traditional stereotactic method. The option is designed to provide a more efficient workflow that reduces the exam time.
“We want to make sure radiologists are able to provide this option to target lesions that were previously undetectable on tomo biopsy,” Banner said.
GE Healthcare
The novel coronavirus pandemic has also changed how companies provide service and train customers on its equipment. GE Healthcare has been continuously improving capabilities of its OnWatch preventive maintenance software, and has been piloting a virtual online trainer for mammographers.
“Our goal is to help our customers with this tremendous backlog they will be facing,” said Agnes Berzsenyi, president and chief executive officer of women’s health for GE Healthcare.
Last year, GE Healthcare and healthcare improvement company Premier Inc. announced they would be collaborating to develop a model for a same-day breast cancer diagnosis and treatment model, with streamlined screening and same-day biopsies.
“With our Pristina Serena, we can transform a screening room to an interventional suite in a few minutes,” Berzsenyi said.
Hologic
Over the last two years, Hologic has made a few acquisitions, including of digital specimen company Faxitron Bioptics, bioresorbable tissue marker developer Focal Therapeutics and ultrasound manufacturer SuperSonic Imagine.
The Supersonic Imagine acquisition in August of last year provided Hologic with an entrée into the ultrasound market using the company’s Aixplorer and MACH 30 platform.
“Our global reach and footprint will create new commercial opportunities with these platforms,” said Samir Parikh, the global vice president of research and development for Hologic.
Last November, the FDA approved Hologic’s 3DQuorum Imaging Technology powered by Genius AI, which works with Hologic’s Clarity HD high resolution imaging technology to reduce tomosynthesis image volume for radiologists by 66%. This results in improved read time.
“You can reduce almost an hour of an eight-hour workday,” Parikh said.
Also last November, the company debuted its Unifi Workspace, which integrates the reading of MR, ultrasound and mammography into one platform, which Parikh said also improved workflow efficiency.
Radiologists also get personalized hanging protocols when they’re logged in.
iCAD
iCAD has continued improving the overall sensitivity and specificity performance of its ProFound AI software, which was FDA cleared in December 2018. The software aids in the detection of breast cancer in digital breast tomosynthesis and assists in clinical decision-making and improving reading efficiency, providing radiologists with Certainty of Finding lesion and Case Scores.
It is also focusing on further enhancing the ProFound AI algorithm by adding the ability to analyze a woman’s prior mammography images to better determine the likelihood of malignancy.
"In other words, ProFound AI will be able to determine if a mass or calcification cluster has changed since the last mammogram, which can be an important indication of cancer," said Michael Klein, chairman and chief executive officer of iCAD.
The software, which the company says provides a 52.7% reduction in reading time for radiologists, will help resolve the issue of prioritizing patients in the wake of the COVID-19 pandemic, Klein said.
In late 2020, iCAD will be launching a short-term risk assessment solution that can help determine a women’s risk of developing cancer prior to her next screening. The solution, ProFound AI Risk, was developed in partnership with researchers at the Karolinska Institute in Stockholm, Sweden.
The risk model assesses the risk of developing breast cancer based on the patient’s age and information found in their mammography images. Along with age, the risk model factors in the presence of masses, calcifications, breast density, and their asymmetry to determine the risk of developing breast cancer in the next one or two years or before the next screening.
A large retrospective study conducted in Sweden showed that the risk model significantly outperforms traditional risk models that are based on family history, lifestyle factors and genetics when determining short-term risk, Klein said.
"We believe this new risk model will be a catalyst in the long-overdue transition from age-based screening to risk-based screening," Klein said. "This product will offer a more holistic clinical approach that can provide clinicians with a broader view of each individual patient’s case and short-term breast cancer risk. ... We believe this AI-centric panoramic view of a woman will lead to even earlier cancer detections, as well as a much more appropriate utilization of breast cancer screening resources including both supplemental screening and screening frequency."
The product will first be available for full-field digital mammography with a primary focus on the EU market in the third quarter of 2020. In early 2021, the company plans to release a version of ProFound AI Risk for digital breast tomosynthesis.
Ikonopedia
Ikonopedia offers a cloud-based structured breast reporting system known as the Ikonopedia Breast Reporting and Tracking software, as well as a tablet-based patient questionnaire that employs a web-based version of the Tyrer-Cuzick Breast Cancer risk assessment tool.
The patient questionnaire was recently updated to include any language — one customer, the Mount Sinai Health System in New York City, uses 14 different languages — which helps to reduce the cost of translation services.
“(Providers have) also seen that the follow-up care has been higher, given that all patients receive their results and reminder letters in their native language,” said Emily Crane, Ikonopedia's chief executive officer.
Ikonopedia also utilizes competing mortality in the Tyrer Cuzick risk assessment that creates the most specific breast cancer risk possible.
“As I age, the other things that could cause my death become higher,” Crane explained. “What it allows them to do and spend their healthcare dollars more wisely.”
As part of its Breast Reporting and Tracking software, Ikonopedia also has a tool that can track images for MQSA-Enhancing Quality Using the Inspection Program (EQUIP) audits, which evaluates image quality.
“We developed an EQUIP tool so they can actually track and audit those images in an automated way,” Crane said. “We also create all reports that are needed in the breast center including MQSA, EQUIP, and timeliness of follow up care.
Koios Medical Inc.
Last July, Koios announced FDA clearance of its Koios DS Breast 2.0, AI-based decision support software that assists radiologists in analyzing and classifying suspicious breast ultrasound images.
The software provides an AI-generated finding, providing a probability of malignancy within the appropriate BI-RADS category and descriptors for reporting.
“When using ultrasound, something is suspicious,” said Chad McClennan, president and chief executive officer of Koios. “Koios DS is an instantaneous second opinion for those cases.”
The software’s ensemble of algorithms have been trained on hundreds of thousands of images from a variety of patient demographic profiles derived from a wide range of ultrasound system manufacturers, and McClennan says customers have seen workflow improvements combined with a reduction in benign biopsies and avoidable, unnecessary follow-up appointments, while catching more cancers earlier.
Koning
As Koning continues to market its Koning Breast CT (KBCT) system, the company recently applied for six new Current Procedural Terminology (CPT) codes and anticipates an announcement from the American Medical Association sometime after press time.
New CPT codes provide the first step toward reimbursement for breast CT in place of a diagnostic mammographic workup, allowing physicians to order exams with or without contrast.
Previous to dedicated codes, facilities were required to use unlisted codes, making reimbursement uncertain, said David Georges, president of the company’s USA division.
Breast CT is also seen as low-cost alternative to MR for breast cancer diagnosis.
“We believe the clinical and payer environment will embrace this technology very quickly,” Georges said.
Medical Scientific
In the second half of 2019, Medical Scientific released a wireless retrofit detector that can be used to upgrade analog mammography systems.
Since the detector is wireless, it is portable and can be moved from one system to another, ideal for a facility with multiple rooms.
“They can effectively upgrade both systems for the cost of one detector,” said Steven Veyland, chief operating officer of Medical Scientific.
The detector is targeted at developing markets in South America, Southeast Asia and Africa, or facilities that have purchased CR systems.
The detector has a 49.5-micron pixel size, versus the 70- to 85-micron pixel size from competitors.
“It provides a much higher image resolution,” Veyland said. “The resulting image they get will be a higher resolution than any 2D system on the market.”
The company also has a mammography reading workstation with a 5 megapixel monitor.
“We provide the complete solution,” Veyland said.
Planmed
The company has improved the algorithm for the synthetic 2D image on its Planmed Clarity 3D digital breast tomosynthesis system
“It looks like a normal 2D image so the radiologist doesn’t have to look at something different,” said Jukka Erkkilä, clinical and product management director of Planmed Oy. “The radiologists really prefer it and sometimes they prefer it over the normal 2D.”
Planmed also had a software update in December that improved post processing for tomosynthesis and stereotactic biopsy.
“It improves tissue separation and also sharpness,” Erkkilä said. “Microcalcifications are clearer.”
The update also provides support for AccuBoost brachytherapy.
Qlarity Imaging
Formerly known as Quantitative Insights, the company was acquired by Paragon Biosciences, a Chicago-based incubator, in June of 2019.
Since then, Qlarity Imaging has made upgrades to the usability and efficiency of its QuantX AI-based decision support software for breast cancer diagnosis using MRI.
The software mimics an app-style workflow, said Robert Tomek, chief technology officer of Qlarity Imaging.
“There are less clicks to get where you need to go,” Tomek said. “We’re putting what the users want right in front of them.”
The company is also researching other breast imaging areas outside of MR, with plans to develop a single platform for all breast imaging modalities in the future.
ScreenPoint Medical
In late 2018, ScreenPoint Medical received FDA clearance for Transpara, its AI application to assist radiologists with reading screening 2D mammograms. In March 2020, ScreenPoint received FDA clearance for use with 3D mammograms — the first AI application to be cleared for use with both 2D and 3D mammograms.
The product, which is distributed in the United States, Australia, New Zealand and parts of Asia by Volpara, provides radiologists with a “second set of eyes,” said David Lee, ScreenPoint Medical’s marketing manager.
The company said customers are also eyeing the solution to help them with the backlog of screening exams due to the COVID-19 pandemic.
Siemens Healthineers
The company has a software update for its MAMMOMAT Revelation that has just received FDA clearance. Called VC20, the software will include efficiency boosts, including faster calibration of the system — from 76 minutes down to five minutes — as well as a faster booting time of four minutes versus 20 minutes and 50-degreeTomoFlow, a feature that permits faster exam times.
With the 50-degree TomoFlow, the patient doesn’t have to wait in between image captures with a compressed breast, providing a better patient experience, said Sammie Stein, women's health product manager at Siemens Healthineers North America.
“Before, the technologist would take the image and wait,” Stein said. “Now, they can take images back to back and the reconstruction happens in the background.”
Storage on the system has also been doubled and image transfer is more flexible, and can be done after each exam or each exposure.
“There is flexibility in what works best for their practice,” Stein says.
The company also plans to release new accessories for biopsies, including a spacer plate that allows for easier imaging of smaller breasts, allowing for less space between the compression panel and the table, and a lateral needle holder for easier lateral targeting.
Siemens Healthineers also released an upgrade to its XA20 software for the BioMatrix family of MR scanners, including the 3T MAGNETOM Vida and Lumina systems and the 1.5T MAGNETOM Sola and Altea scanners.
The update includes an AI workflow enhancement for breast biopsy imaging, with automatic calculation to improve needle positioning together with the Select&GO feature to see target positioning information on the scanner itself.
“Before, you would have to plan and target each lesion individually, moving between the scan room and control room to take each biopsy,” said Kelly Parker, senior product marketing manager for MR at Siemens Healthineers North America. “Now you can plan the targets for all biopsies at the same time, then return to the scan room to perform the biopsies, resulting in a more streamlined workflow for the clinician and the patient.”
Volpara
Last year, Volpara Solutions acquired MRS Systems Inc., a software company with a reporting system that allows healthcare providers to document patient history, risk factors and radiology findings from breast screening exams.
Mark Koeniguer, chief commercial officer and president of the U.S. division of Volpara, said the merger brings together VolparaRisk software, a breast cancer risk assessment software, and Aspen Breast product, marking a shift from traditional pure screening to risk stratified screening.
“With the acquisition of MRS last year … we can fundamentally change how women are triaged to provide supplemental imaging based on risk,” Koeniguer said. “If they are dense but not high-risk, they might be a candidate for ultrasound and not MR. Whereas, a woman at high risk may be a candidate for MR despite other factors. The two (products) fit together and speak to one another.”
Customers of the VolparaPlatform, which was launched at last year’s RSNA, include customers across the imaging spectrum, from small imaging centers to large IDNs, and it has increased breast cancer detection rates.
“A lot of this information existed in various forms in different parts of the department, but it wasn’t easily accessible during the reading of the mammogram,” Koeniguer said. “There are sites using this and their detection rates are higher.”