What’s new in MR scanners and coils?
October 16, 2017
by Lisa Chamoff, Contributing Reporter
Hospitals and imaging centers all have the same goals when performing MR exams: increasing patient satisfaction and achieving good outcomes while containing costs.
To that end, MR scanner and coil manufacturers are releasing new products with these goals in mind, allowing for faster scanning speeds with clear images, and coils and other products to make an often-uncomfortable and anxiety-producing exam as comfortable as possible.
Here’s a guide to the newest MR products that have come out within the last year:
Alltech Medical Systems/Swissray
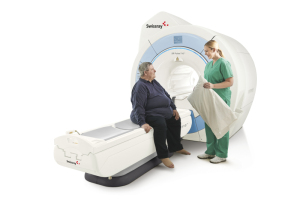
In April, Alltech received FDA clearance for the 4.0 software upgrade for the EchoStar Comfort 1.5T wide-bore MR scanner. The EchoStar Comfort is sold throughout the world, including Asia and the Middle East.
In the U.S. and Europe, Alltech formed a strategic partnership with Swissray, which has exclusive rights to sell the system as the SR Pulse 710. The system has the widest internal bore diameter at 71 centimeters, a patient table weight limit of 550 pounds, and addresses neuro, orthopedic and body MR imaging.
Swissray has one beta install up and running and is installing another system at a new site in October, says Anne Sheehan, director of MRI for Swissray International, Inc.
"The market has a gap,” Sheehan says. “Most new 1.5 Tesla systems run around $1 million. For customers who can't afford that price point, there simply is not a supply of used wide bore systems to fill that need. The SR Pulse is perfectly poised to fill that gap, with a 71 centimeter bore, a full range of coils and great throughput potential at a price that competes with refurbished wide bore systems. Our system could also complement an institution with more than one MR, serving as a workhorse system that handles the bread and butter applications — brain, spine and orthopedics — that account for much of our customers' daily routine."
Swissray, long a major player in the digital radiography space, has expanded its sales team to include people with experience in MR sales, and hired a third-party service organization while the company trains its DR service engineers in MR.
GE Healthcare
GE Healthcare recently received FDA clearance for its SIGNA Premier, the company’s latest 3T wide bore scanner. The system offers additional research-focused capabilities, particularly for neurology and oncology research, says Michael Brandt, chief marketing officer for GE Healthcare MR.
The SIGNA Premier is powered by a SuperG gradient coil that is designed to deliver the image quality of 60cm MR systems normally used for research purposes, but with a 70 centimeter bore that can accommodate larger patients and those who are claustrophobic.
“This product features GE Healthcare’s latest, short-bore, high-homogeneity 3T superconductive magnet, the most powerful gradient system GE Healthcare has ever developed for a wide bore 3T system, and a new, digital RF transmit and receive architecture,” Brandt says.
GE has also applied for 510(k) clearance for Air Coil, a new suite of coils for the SIGNA Premier. The coils are 66 percent lighter and consume half the power of other coils, Brandt says. The copper loop etching process has also been eliminated, resulting in a “green” solution.
“These new coils are going to be a huge differentiator for patient comfort, but also for workflow and for the techs,” Brandt says. “Coils tend to be bulky, susceptible to drops and breakage, and hard to fit to some anatomies and patients. But Air Coil is like a blanket. It’s lightweight and can adapt easily to different patients. Because the coils wrap around the anatomy of interest, it's great for receiving signal because it can get closer to the anatomy of interest.”
In September 2016, GE received FDA clearance for MAGiC, which the company says is the industry’s first multi-contrast MR imaging technique. Used for neurological imaging, the technique gives clinicians more data than conventional scanning in a fraction of the time.
“MAGiC gives users the flexibility to manipulate images retrospectively, leading to significant time savings, fewer rescans and potentially cost savings,” Brandt says.
Another new software offering from GE is HyperWorks, which is part of the SIGNAWorks Innovation applications available on the SIGNA Architect 3T system and SIGNA Artist 1.5T system, both made commercially available in April, as well as the SIGNA Premier, SIGNA Pioneer and with systems that have upgraded to the latest platform.
HyperSense, part of HyperWorks, is a new compressed sensing application that allows clinicians to reduce scan times or increase scan resolution. It can deliver up to eight times faster results in brain, head and neck, spine, musculoskeletal, abdominal and pelvic imaging, Brandt says.
The software works by sampling sparse data and then performing iterative reconstruction. It can also be combined with other methods, such as ARC, for achieving high signal-to-noise ratio with shorter acquisition times, according to Brandt.
Bayer Radiology
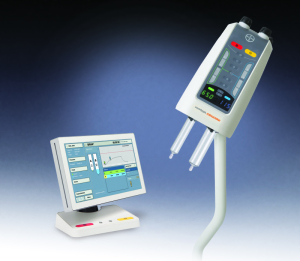
Bayer Radiology’s newest MR product is the MRXperion, a contrast injection system. The product is a smart injection system designed for contrast delivery and contrast dose management through its integration with the Radimetrics Enterprise Platform, which is the first injection system that is seamlessly integrated with an informatics platform, says Dennis Durmis, head of the Americas region for radiology at Bayer.
The smart injection system improves the accuracy of the data related to contrast delivery, Durmis says.
Usually, MR technologists need to write down what type of contrast was used and how much was delivered to the patient. With the MRXperion, the injector has access to the patient work list, which has information on the patient and the exam.
“The patient information can all be accessed from the informatics platform,” Durmis says. “Having the electronic record brings confidence for the injection of which patient is being imaged and which area of the body is being imaged. The Radimetrics Enterprise Platform provides modality work list connectivity and personalized patient-specific protocols and data capture, taking away the need for manual entry and reducing mistakes.”
This system also allows technologists to set up for the exam more quickly, Durmis says.
The system can be used with any scanner and patient information system, and any type of contrast can be used.
The company had a controlled commercial release and received positive feedback.
“It’s perceived as a next-generation product,” Durmis says. “Clearly, the trend is for more interconnected devices. The idea of having a standalone unit in a hospital is becoming antiquated.”
The device also allows for Bayer to be more predictive and proactive when it comes to service of the injection system, part of Bayer's Radimetrics Enterprise Platform.
“We can see ahead of time what’s failing and what we need to do so that we can ensure there’s uptime in the suite,” Durmis says.
NeoCoil
NeoCoil is in the process of submitting an application for FDA 510(k) clearance for a new 16-channel breast coil for 1.5T and 3T MR scanners.
Aside from producing high-quality images, the new coil is lightweight and can be altered to fit different breast sizes, fitting 99 percent of the population, says Venkat Goruganti, director of RF coil development at NeoCoil.
The coil was designed to be easily adjustable for maximum patient comfort.
“Patient comfort has been critical for breast imaging,” Goruganti says.
The coil also has an open access design for diagnostic scans, as well as lateral and medial biopsies. Coils come with configurations for a 16-channel bilateral diagnostic, eight-channel bilateral biopsy, 12-channel lateral biopsy and six-channel medial biopsy.
The coil comes with a narrow width in the medial area, which helps to keep the breast anatomy closer to its natural shape.
“Other designs have a wider medial area, which keep the breasts too far apart, and you lose the natural shape, which doctors don’t prefer,” Goruganti says.
NeoCoil recently received FDA clearance for its Sentinel Wireless Audio System, which allows patients to listen to music or other audio during MR imaging while drowning out the noise of the scanner. It also integrates with the scanner so music can be interrupted by the operating technician.
Other MR headphones rely on the use of pneumatic tubes, says Brian Brown, acting president and chief innovation officer at NeoCoil.
“We feel that these wireless headphones are the new way of the future in terms of improving workflow,” Brown says.
Philips
At RSNA 2016, Philips released a new suite of software applications dedicated to neurology, for use with its Ingenia digital MR systems. These eight applications include six on the scanner itself and two on the Philips IntelliSpace Portal, an advanced visualization and analysis solution that offers a simple approach to streamline workflow.
“We talked to a lot of neuroradiologists,” says Martijn Hartjes, global product marketing manager for MR at Philips. “They are looking to answer complex questions in traumatic brain injury and more advanced disease.”
Included among the applications within the scanner is MultiBand SENSE, which allows for multiple slices of the brain to be acquired simultaneously during fMR exams, without a longer scan time.
“This technology was used before in [the] research domain,” Hartjes says.
Another application, called 4-D-TRANCE, allows for visualization of inflammation in the vessel wall of the brain without contrast for an easier diagnosis of vasculitis.
The goal is for Philips to help its customers reduce costs by increasing scanning speeds and reducing rescans, as well as make better diagnoses and treatment decisions, thereby lowering readmission rates.
“That trickles down to other parts of the equation in hospital costs,” Hartjes says.
A few months ago, Philips released a new set of musculoskeletal coils designed to improve image quality and coverage and fit more patients. The design of the coils allows them to tolerate higher acceleration speeds, Hartjes says.
ScanMed
This past summer ScanMed, which manufactures MR coils, unveiled an innovative pediatric brain coil set, with coils of three different sizes that can be used to scan premature infants to children up to age 18. Clinicians can switch out the small, medium and large head coils without having to reconnect the base to the MR machine, says Natalie Hussey, ScanMed’s marketing manager.
“If the coil doesn’t fit, you can quickly switch it out,” Hussey says.
Previously, coils were made for either children or adults.
“It was sort of a one-size-fits-all approach, which doesn’t produce the quality of images that you need to diagnose,” Hussey says. “None of them were optimized for the size of the child.”
Neurological scanning is becoming more common for children, especially with more attention being paid to football and other sports-related injuries.
The company also recently unveiled a dedicated eight- and 16-channel wrist and ankle coil.
The coils are compatible with GE and Siemens MR scanners. ScanMed is working with Philips on compatibility, and all are pending FDA clearance. The company is looking for demo sites to improve and optimize the pediatric coils.
ScanMed is also working with Duke University to investigate using a dual-tuned, multinuclear coil to diagnose chronic obstructive pulmonary disease.
“Hydrogen is the best atom to image with overall because it is present in all tissue and has a net nuclear spin,” Hussey says. “However, hydrogen isn't the best atom for imaging certain organs and pathologies. In the case of lung imaging, because patients need to breathe, it's hard to get good images with just hydrogen because the movement disrupts the acquisition process, so the images are often blurry. Our coil utilizes a gas mixture of oxygen, which allows for free-breathing, and 19F is an inert gas that distributes quickly through the lungs and transmits a bright signal, making it so we can image in real time without the blurriness used by hydrogen.
“We hope to then take this technology and apply it to other areas of the body where it is difficult to get great images with hydrogen alone without clinics having to invest in expensive multinuclear systems.”
Siemens Healthineers
In June, Siemens Healthineers received FDA clearance for its newest 3T MR system, the MAGNETOM Vida. The system features the company's new BioMatrix technology, which takes into account the physiological and anatomical differences between patients, including size and sex, providing a more personalized approach to MR scanning.
The BioMatrix technology includes head/neck coils designed with what Siemens calls CoilShim to reduce variability in image quality based on patient anatomy.
“If you want to get consistent images, you need to recognize that variability and adjust your imaging,” says Heather Lewis, MR marketing director for Siemens Healthineers. “Previous MR systems have not addressed targeted regions in this way. For this reason, they were not as precise.”
BioMatrix also comes with respiratory sensors that automatically provide information on a patient’s breathing before imaging starts. This information can be used in combination with the scanner's many day optimizing throughput (DOT) engines to accelerate the exam.
On MAGNETOM Vida, Siemens also introduced new coils that were designed to improve positioning and enhance patient comfort. For example, the system's dedicated knee coil has an expanded diameter and flares toward the thigh to accommodate a greater number of patients.
Toshiba
At RSNA 2016, Toshiba unveiled its Vantage Galan 3T MR system, building on the company's 3T Titan MR, introduced six years ago.
The Vantage Galan, positioned for facilities with high throughput, has a 71 centimeter bore. It comes with Toshiba’s new Pianissimo Zen acoustic noise reduction technology which, through a combination of hardware and software features, can reduce acoustic noise by more than 99 percent, says Jon Furuyama, senior manager of market development for MR at Toshiba.
As sound is transmitted through air, Toshiba’s engineers took the gradient coil and enclosed it in a vacuum chamber. Furuyama says the bore does taper down to support Pianissimo technology, but the change is worth it for the increase in patient comfort with noise reduction.
“The amount of sound that gets to the patient is dramatically reduced,” Furuyama says. “It is remarkable how quiet we’re able to get the scanner. It’s to the point where people don’t realize the system is acquiring data.”
The Pianissimo Zen software is also available to purchase as an upgrade on Toshiba’s Vantage Titan 1.5T MR scanner.
Also available for the Galan and Titan is Toshiba’s new MR Theater, a screen that fits inside the MR bore and, visible with a mirror, lets patients watch TV and provides a distraction from the often anxiety-producing exam.
Other manufacturers place screens outside the bore, according to Furuyama.
On other systems, patients have to “look pretty far,” Furuyama says. “This screen is sitting right behind the patient, so it’s designed to give an immersive experience. They really lose this feeling that they’re moving inside a bore.”
Toshiba’s Pianissimo Zen and MR Theater offerings are designed to provide a better patient experience.
“Customers are being more evaluated for how a patient feels,” Furuyama says. “We feel that this technology gives the best patient experience out there.”
To that end, MR scanner and coil manufacturers are releasing new products with these goals in mind, allowing for faster scanning speeds with clear images, and coils and other products to make an often-uncomfortable and anxiety-producing exam as comfortable as possible.
Here’s a guide to the newest MR products that have come out within the last year:
Alltech Medical Systems/Swissray
In April, Alltech received FDA clearance for the 4.0 software upgrade for the EchoStar Comfort 1.5T wide-bore MR scanner. The EchoStar Comfort is sold throughout the world, including Asia and the Middle East.
In the U.S. and Europe, Alltech formed a strategic partnership with Swissray, which has exclusive rights to sell the system as the SR Pulse 710. The system has the widest internal bore diameter at 71 centimeters, a patient table weight limit of 550 pounds, and addresses neuro, orthopedic and body MR imaging.
Swissray has one beta install up and running and is installing another system at a new site in October, says Anne Sheehan, director of MRI for Swissray International, Inc.
"The market has a gap,” Sheehan says. “Most new 1.5 Tesla systems run around $1 million. For customers who can't afford that price point, there simply is not a supply of used wide bore systems to fill that need. The SR Pulse is perfectly poised to fill that gap, with a 71 centimeter bore, a full range of coils and great throughput potential at a price that competes with refurbished wide bore systems. Our system could also complement an institution with more than one MR, serving as a workhorse system that handles the bread and butter applications — brain, spine and orthopedics — that account for much of our customers' daily routine."
Swissray, long a major player in the digital radiography space, has expanded its sales team to include people with experience in MR sales, and hired a third-party service organization while the company trains its DR service engineers in MR.
GE Healthcare
GE Healthcare recently received FDA clearance for its SIGNA Premier, the company’s latest 3T wide bore scanner. The system offers additional research-focused capabilities, particularly for neurology and oncology research, says Michael Brandt, chief marketing officer for GE Healthcare MR.
The SIGNA Premier is powered by a SuperG gradient coil that is designed to deliver the image quality of 60cm MR systems normally used for research purposes, but with a 70 centimeter bore that can accommodate larger patients and those who are claustrophobic.
“This product features GE Healthcare’s latest, short-bore, high-homogeneity 3T superconductive magnet, the most powerful gradient system GE Healthcare has ever developed for a wide bore 3T system, and a new, digital RF transmit and receive architecture,” Brandt says.
GE has also applied for 510(k) clearance for Air Coil, a new suite of coils for the SIGNA Premier. The coils are 66 percent lighter and consume half the power of other coils, Brandt says. The copper loop etching process has also been eliminated, resulting in a “green” solution.
“These new coils are going to be a huge differentiator for patient comfort, but also for workflow and for the techs,” Brandt says. “Coils tend to be bulky, susceptible to drops and breakage, and hard to fit to some anatomies and patients. But Air Coil is like a blanket. It’s lightweight and can adapt easily to different patients. Because the coils wrap around the anatomy of interest, it's great for receiving signal because it can get closer to the anatomy of interest.”
In September 2016, GE received FDA clearance for MAGiC, which the company says is the industry’s first multi-contrast MR imaging technique. Used for neurological imaging, the technique gives clinicians more data than conventional scanning in a fraction of the time.
“MAGiC gives users the flexibility to manipulate images retrospectively, leading to significant time savings, fewer rescans and potentially cost savings,” Brandt says.
Another new software offering from GE is HyperWorks, which is part of the SIGNAWorks Innovation applications available on the SIGNA Architect 3T system and SIGNA Artist 1.5T system, both made commercially available in April, as well as the SIGNA Premier, SIGNA Pioneer and with systems that have upgraded to the latest platform.
HyperSense, part of HyperWorks, is a new compressed sensing application that allows clinicians to reduce scan times or increase scan resolution. It can deliver up to eight times faster results in brain, head and neck, spine, musculoskeletal, abdominal and pelvic imaging, Brandt says.
The software works by sampling sparse data and then performing iterative reconstruction. It can also be combined with other methods, such as ARC, for achieving high signal-to-noise ratio with shorter acquisition times, according to Brandt.
Bayer Radiology
Bayer Radiology’s newest MR product is the MRXperion, a contrast injection system. The product is a smart injection system designed for contrast delivery and contrast dose management through its integration with the Radimetrics Enterprise Platform, which is the first injection system that is seamlessly integrated with an informatics platform, says Dennis Durmis, head of the Americas region for radiology at Bayer.
The smart injection system improves the accuracy of the data related to contrast delivery, Durmis says.
Usually, MR technologists need to write down what type of contrast was used and how much was delivered to the patient. With the MRXperion, the injector has access to the patient work list, which has information on the patient and the exam.
“The patient information can all be accessed from the informatics platform,” Durmis says. “Having the electronic record brings confidence for the injection of which patient is being imaged and which area of the body is being imaged. The Radimetrics Enterprise Platform provides modality work list connectivity and personalized patient-specific protocols and data capture, taking away the need for manual entry and reducing mistakes.”
This system also allows technologists to set up for the exam more quickly, Durmis says.
The system can be used with any scanner and patient information system, and any type of contrast can be used.
The company had a controlled commercial release and received positive feedback.
“It’s perceived as a next-generation product,” Durmis says. “Clearly, the trend is for more interconnected devices. The idea of having a standalone unit in a hospital is becoming antiquated.”
The device also allows for Bayer to be more predictive and proactive when it comes to service of the injection system, part of Bayer's Radimetrics Enterprise Platform.
“We can see ahead of time what’s failing and what we need to do so that we can ensure there’s uptime in the suite,” Durmis says.
NeoCoil
NeoCoil is in the process of submitting an application for FDA 510(k) clearance for a new 16-channel breast coil for 1.5T and 3T MR scanners.
Aside from producing high-quality images, the new coil is lightweight and can be altered to fit different breast sizes, fitting 99 percent of the population, says Venkat Goruganti, director of RF coil development at NeoCoil.
The coil was designed to be easily adjustable for maximum patient comfort.
“Patient comfort has been critical for breast imaging,” Goruganti says.
The coil also has an open access design for diagnostic scans, as well as lateral and medial biopsies. Coils come with configurations for a 16-channel bilateral diagnostic, eight-channel bilateral biopsy, 12-channel lateral biopsy and six-channel medial biopsy.
The coil comes with a narrow width in the medial area, which helps to keep the breast anatomy closer to its natural shape.
“Other designs have a wider medial area, which keep the breasts too far apart, and you lose the natural shape, which doctors don’t prefer,” Goruganti says.
NeoCoil recently received FDA clearance for its Sentinel Wireless Audio System, which allows patients to listen to music or other audio during MR imaging while drowning out the noise of the scanner. It also integrates with the scanner so music can be interrupted by the operating technician.
Other MR headphones rely on the use of pneumatic tubes, says Brian Brown, acting president and chief innovation officer at NeoCoil.
“We feel that these wireless headphones are the new way of the future in terms of improving workflow,” Brown says.
Philips
At RSNA 2016, Philips released a new suite of software applications dedicated to neurology, for use with its Ingenia digital MR systems. These eight applications include six on the scanner itself and two on the Philips IntelliSpace Portal, an advanced visualization and analysis solution that offers a simple approach to streamline workflow.
“We talked to a lot of neuroradiologists,” says Martijn Hartjes, global product marketing manager for MR at Philips. “They are looking to answer complex questions in traumatic brain injury and more advanced disease.”
Included among the applications within the scanner is MultiBand SENSE, which allows for multiple slices of the brain to be acquired simultaneously during fMR exams, without a longer scan time.
“This technology was used before in [the] research domain,” Hartjes says.
Another application, called 4-D-TRANCE, allows for visualization of inflammation in the vessel wall of the brain without contrast for an easier diagnosis of vasculitis.
The goal is for Philips to help its customers reduce costs by increasing scanning speeds and reducing rescans, as well as make better diagnoses and treatment decisions, thereby lowering readmission rates.
“That trickles down to other parts of the equation in hospital costs,” Hartjes says.
A few months ago, Philips released a new set of musculoskeletal coils designed to improve image quality and coverage and fit more patients. The design of the coils allows them to tolerate higher acceleration speeds, Hartjes says.
ScanMed
This past summer ScanMed, which manufactures MR coils, unveiled an innovative pediatric brain coil set, with coils of three different sizes that can be used to scan premature infants to children up to age 18. Clinicians can switch out the small, medium and large head coils without having to reconnect the base to the MR machine, says Natalie Hussey, ScanMed’s marketing manager.
“If the coil doesn’t fit, you can quickly switch it out,” Hussey says.
Previously, coils were made for either children or adults.
“It was sort of a one-size-fits-all approach, which doesn’t produce the quality of images that you need to diagnose,” Hussey says. “None of them were optimized for the size of the child.”
Neurological scanning is becoming more common for children, especially with more attention being paid to football and other sports-related injuries.
The company also recently unveiled a dedicated eight- and 16-channel wrist and ankle coil.
The coils are compatible with GE and Siemens MR scanners. ScanMed is working with Philips on compatibility, and all are pending FDA clearance. The company is looking for demo sites to improve and optimize the pediatric coils.
ScanMed is also working with Duke University to investigate using a dual-tuned, multinuclear coil to diagnose chronic obstructive pulmonary disease.
“Hydrogen is the best atom to image with overall because it is present in all tissue and has a net nuclear spin,” Hussey says. “However, hydrogen isn't the best atom for imaging certain organs and pathologies. In the case of lung imaging, because patients need to breathe, it's hard to get good images with just hydrogen because the movement disrupts the acquisition process, so the images are often blurry. Our coil utilizes a gas mixture of oxygen, which allows for free-breathing, and 19F is an inert gas that distributes quickly through the lungs and transmits a bright signal, making it so we can image in real time without the blurriness used by hydrogen.
“We hope to then take this technology and apply it to other areas of the body where it is difficult to get great images with hydrogen alone without clinics having to invest in expensive multinuclear systems.”
Siemens Healthineers
In June, Siemens Healthineers received FDA clearance for its newest 3T MR system, the MAGNETOM Vida. The system features the company's new BioMatrix technology, which takes into account the physiological and anatomical differences between patients, including size and sex, providing a more personalized approach to MR scanning.
The BioMatrix technology includes head/neck coils designed with what Siemens calls CoilShim to reduce variability in image quality based on patient anatomy.
“If you want to get consistent images, you need to recognize that variability and adjust your imaging,” says Heather Lewis, MR marketing director for Siemens Healthineers. “Previous MR systems have not addressed targeted regions in this way. For this reason, they were not as precise.”
BioMatrix also comes with respiratory sensors that automatically provide information on a patient’s breathing before imaging starts. This information can be used in combination with the scanner's many day optimizing throughput (DOT) engines to accelerate the exam.
On MAGNETOM Vida, Siemens also introduced new coils that were designed to improve positioning and enhance patient comfort. For example, the system's dedicated knee coil has an expanded diameter and flares toward the thigh to accommodate a greater number of patients.
Toshiba
At RSNA 2016, Toshiba unveiled its Vantage Galan 3T MR system, building on the company's 3T Titan MR, introduced six years ago.
The Vantage Galan, positioned for facilities with high throughput, has a 71 centimeter bore. It comes with Toshiba’s new Pianissimo Zen acoustic noise reduction technology which, through a combination of hardware and software features, can reduce acoustic noise by more than 99 percent, says Jon Furuyama, senior manager of market development for MR at Toshiba.
As sound is transmitted through air, Toshiba’s engineers took the gradient coil and enclosed it in a vacuum chamber. Furuyama says the bore does taper down to support Pianissimo technology, but the change is worth it for the increase in patient comfort with noise reduction.
“The amount of sound that gets to the patient is dramatically reduced,” Furuyama says. “It is remarkable how quiet we’re able to get the scanner. It’s to the point where people don’t realize the system is acquiring data.”
The Pianissimo Zen software is also available to purchase as an upgrade on Toshiba’s Vantage Titan 1.5T MR scanner.
Also available for the Galan and Titan is Toshiba’s new MR Theater, a screen that fits inside the MR bore and, visible with a mirror, lets patients watch TV and provides a distraction from the often anxiety-producing exam.
Other manufacturers place screens outside the bore, according to Furuyama.
On other systems, patients have to “look pretty far,” Furuyama says. “This screen is sitting right behind the patient, so it’s designed to give an immersive experience. They really lose this feeling that they’re moving inside a bore.”
Toshiba’s Pianissimo Zen and MR Theater offerings are designed to provide a better patient experience.
“Customers are being more evaluated for how a patient feels,” Furuyama says. “We feel that this technology gives the best patient experience out there.”